The influence of electrolytic Zn–Ni alloys structural characteristics on their electrochemical properties
DOI:
https://doi.org/10.15421/081421Keywords:
zinc-nickel, electrolytic alloys, structural characteristics, anodic behavior, alkaline mediumAbstract
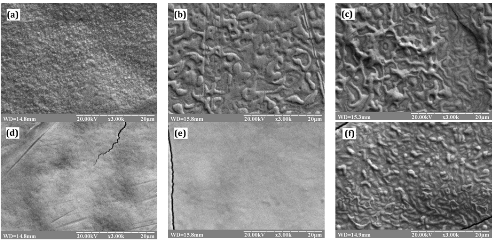
In this work the effects of structural characteristics of electrolytic Zn–1.0 wt% Ni alloys on their anodic behavior in alkaline solutions were investigated. Electrolytic alloys deposited from five zincate electrolytes which differed from one another by ligand composition. Phase composition of Zn–Ni alloys was determined by X-ray diffraction and electron microscopy analysis. The crystallites sizes, microstresses and dislocation density coating of zinc and Zn–Ni alloys were identified. The morphology of the surface plating was studied using scanning electron microscope. It is shown that the primary factor that determines the electrochemical properties of the alloys is their phase composition. Other structural characteristics (morphology, texture, size, shape and defects of crystallite) have not significant influence on the course of the anode current-voltage characteristics in the alkaline solution. They can only affect the speed of metal dissolution and anodic peaks.
References
Hu, H. L., Zhu, Y. M., Tu, Z. M., Liu, W. J. High anti-corrosion nano Zn-Fe coatings by pulse electrodepositing. Adv. Mat. Res., 2011, vol. 194-196, p. 2209–2215.
Boshkov, N., Tsvetkova, N., Petrov, P., Koleva, D., Petrov, K., Avdeev, G., Tsvetanov, Ch., Raichevsky, G., Raicheff, R. Corrosion behavior and protective ability of Zn and Zn-Co electrodeposits with embedded polymeric nanoparticles. Appl. Surf. Sci., 2008, vol. 254, p. 5618–5625.
Baldwin, K. R., Smith, C. J. E., Robinson, M. J. Cathodic protection of steel by electrodeposited zinc-nickel alloy coatings. Corros., vol. 51, p. 932–940.
Tian, W., Xie, F. Q., Wu, X. Q., Yang, Z. Z. Study on corrosion resistance of electroplating zinc-nickel alloy coatings. Surf. Interface. Anal., 2009, vol. 41, p. 251–254.
Ashiru, O. A., Shirokoff, J. Electrodeposition and characterization of tin-zinc alloy coatings. Appl. Surf. Sci., 1996, vol. 103, p. 159–169.
Fontenay, F. Electrodeposited zinc and zinc alloy coatings and their corrosion resistance. Part 1. Galvanotechnik, 2002, vol. 93, p. 2534–2541.
Shibuya, A., Kurimoto, T., Korekawa, K., Noji, K. Corrosion-resistance of electroplated Ni-Zn alloy steel sheet. Tetsu-to-Hagané, 1980, vol. 66, no. 7, p. 771–778.
Tsybulskaya, L. S., Gaevskaya, T. V., Purovskaya, O. G., Byk T. V. Electrochemical deposition of zinc-nickel alloy coatings in a polyligand alkaline bath. Surf. Coat. Technol., 2008, vol. 203, no. 3-4, p. 234–239.
Mahieu, J., De Wit, K., De Boeck, A., De Cooman, B. C. The properties of electrodeposited Zn-Co coatings. J. Mater. Eng. Perform., 1999, vol. 8, no. 5, p. 561–570.
Park, H., Szpunar, J. A. The role of texture and morphology in optimizing the corrosion resistance of zinc-based electrogalvanized coatings. Corros. Sci., 1998, vol. 40, no. 4, p. 525–545.
Gharahcheshmeh, M. H., Sohi, M. H. Electrochemical studies of zinc-cobalt alloy coatings deposited from alkaline baths containing glycine as complexing agent. J. Appl. Electrochem., 2010, vol. 40, no. 8, p. 1563–1570.
Albalat, R., Gómez, E., Müller, C., Sarret, M., Vallés, E., Pregonas, J. Electrodeposition of zinc-nickel alloy coatings: influence of a phenolic derivative. J. Appl. Electrochem., 1990, vol. 20, p. 635–639.
Prentice, G., Chang, Y. C., Shan, X. Model for the passivation of the zinc electrode in alkaline electrolyte. J. Electrochem. Soc., 1991, vol. 138, p. 890–894.
Ramanauskas, R. Structural factor in Zn alloy electrodeposit corrosion. Appl. Surf. Sci., 1999, vol. 153, no. 1, p. 53–64.
Petrenko, L. V., Korobov, V. I. Phase composition of electrolytic Zn-Ni coatings. Visn. Dnipropetr. Univ.: Khim., 2010, no. 16, p. 33–40. [in Ukrainian]
Petrenko, L. V., Bagan, M. Yu., Korobov, V. I. Effect of zincate solution composition on the nickel content in Zn-Ni alloy platings. Visn. Dnipropetr. Univ.: Khim., 2011, no. 17, p. 30–34. [in Ukrainian]
Marchenko, Z., Photometric determination of elements. Moscow: Mir, 1971, 482 p. [in Russian]
Mirkin, L. I. Guide to X-ray analysis of polycrystals. Moscow: Gos. izd-vo fiziko-matemat. lit-ry, 1961, 864 p. [in Russian]
Malykhin, D. G., Kovtun, G. P., Stukalov, A. I., Chernyayeva, T. P., Gritsina, V. M. Investigations of the substructural characteristics of the zirconium alloys by modified approximating method. Vopr. atom. nauki i tekhn.: Fizika radiats. povrezhd. i radiats. materialoved., 2003, no. 3, p. 117–122. [in Russian]
Gorelik, S. S., Rastorguyev, L. N., Skakov, Yu. A., X-ray and electron-optical analysis. Moscow: Metallurgiya, 1970, 366 p. [in Russian]
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

