Sequential injection spectrophotometric determination of analgine in pharmaceutical formulations using 18-molybdo-2-phosphate heteropoly anion as chromogenic reagent
DOI:
https://doi.org/10.15421/081301Keywords:
analgine, 18-molybdo-2-phosphate, sequential injection analysisAbstract
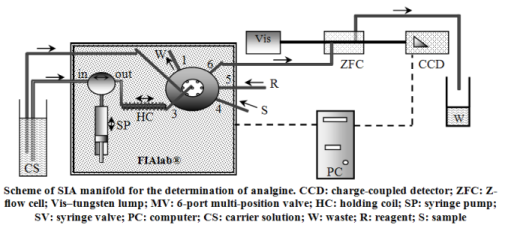
Simple, sensitive and selective sequential injection analysis (SIA) method for the analgine determination has been developed on the basis of fast reaction between analgine and 18-molybdo-2-phosphate heteropoly anion (18-MPA). Under found optimal conditions (0.01 M HCl, C(18-MPA) = 2 mmol/L) linear calibration curve was obtained over the range from 0.5 to 80 mg/L of analgine, and detection limit (S/N = 3) was 0.2 mg/L. The proposed SIA method has high sample throughput of 45 h-1 and small reagent consumption (0.08 mL). The procedure was successfully applied to the analysis of pharmaceuticals.References
Marcolino-Júnior, L.H., Sousa, R.A., Fatibello-Filho, O., Moraes, F.C. Flow-Injection spectrophotometric determination of dipyrone in pharmaceutical formulations using ammonium molybdate as chromogenic reagent. Anal. Lett., 2005, vol. 38, no. 14, p. 2315-2326.
Lima, J.L.F.C., Sá, S.M.O., Santos, J.L.M., Zagatto, E.A.G. Multi-pumping flow system for the spectrophotometric determination of dipyrone in pharmaceutical preparations. J. Pharm. Biomed. Anal., 2003, vol. 32, no. 4, p. 1011-1017.
Srivastava, M.K., Ahmad, S., Singh, D., Shukla, I.C. Titrimetric determination of dipyrone and paracetamol with potassium hexacyanoferrate(III) in an acidic medium. Analyst, 1985, vol. 110, p. 735-737.
Qureshi, S.Z., Saeed, A., Haque, S. Some observations on a simple method for the determination of novalgin in drug formulations with iron(III) – 1,10-phenanthroline. Microchem. J., 1990, vol. 41, no. 3, p. 362-365.
Qureshi, S.Z. Saeed, A., Hasan, T. Spectrophotometric determination of novalgin in tablets by use of potassium iodate. Talanta, 1989, vol. 36, no. 8, p. 869-871.
Sakiara, K.A., Pezza, L., Melios, C.B., Pezza, H.R., Moraes, M. Spectrophotometric determination of dipyrone in pharmaceutical preparations by using chromotropic acid. Il Farmaco, 1999, vol. 54, no. 9, p. 629-635.
Suarez, W.T., Pessoa-Neto, O.D., Vicentini, F.C., Janegitz, B.C., Faria, R.C., Fatibello-Filho, O. Flow Injection Spectrophotometric Determination of Dipyrone in Pharmaceutical Formulations Using Fe(III) as Reagent. Anal. Lett., 2011, vol. 44, no. 1-3, p. 340-348.
Zaporozhets, O.A., Krushinskaya, E.A., Lipkovskaya, N.A., Sukhan, V.V. Solid-phase reagent for analgin and ascorbic acid on the basis of a copper(II) complex with tetrabenzotetraazacyclohexadecine immobilized by adsorption on silica gel. J. Anal. Chem., 2001, vol. 56, no. 6, p. 524-529.
Morelli, B.J., Pharm. Biomed. Determination of binary mixtures of analgesic and spasmolytic drugs in pure and dosage forms by derivative spectrophotometry. Anal., 2003, vol. 33, no 3, p. 423-433.
Pradana Pérez, J.A., Alegría, J.S.D., Hernando, P.F., Sierra, A.N. Determination of dipyrone in pharmaceutical preparations based on the chemiluminescent reaction of the quinolinic hydrazide – H2O2 – vanadium(IV) system and flow injection analysis. Luminescence, 2011, vol. 27, no. 1, p. 45-50.
Zhao, L., Li, B., Zhang, Z., Lin, J.-M. Chemiluminescent flow-through sensor for automated dissolution testing of analgin tablets using manganese dioxide as oxidate. Sensors and Actuators, 2004, vol. 97, no. 2-3, p. 266-271.
Perez-Ruiz, T., Martinez-Lozano, C., Tomas, V., Carpena, J. Flow-injection fluorometric determination of novalgin in pharmaceutical preparations. Microchem. J., 1993, vol. 47, no. 3, p. 296-301.
Basáez, L.A., Periĉ, I.M., Jara, P.A., Soto, C.A., Contreras, D.R., Aguirre, C., Vanysek, P. Electrochemical and electrophoretic study of sodium metamizole. J. Chil. Chem. Soc., 2008, vol. 53, no. 3, p. 1572-1575.
Marcolino-Júnior, L.H., Bergamini, M.F., Teixeira, M.F.S., Cavalheiro, E.T.G., Fatibello-Filho, O. Flow injection amperometric determination of dipyrone in pharmaceutical formulations using a carbon paste electrode. Il Farmaco, 2003, vol. 58, no. 10, p. 999-1004.
Medeiros, E.P., Castro, S.L., Formiga, F.M., Santos, S.R.B., Araujo, M.C.U., Nascimento, V.B. A flow injection method for biamperometric determination of dipyrone in pharmaceuticals. Microchem J., 2004, vol. 78, no. 1, p. 91-96.
Pérez-Ruiz, T., Lozano, C.M., Tomás, V. Flow-injection determination of Novalgin using amperometric detection at a glassy carbon electrode. J. Pharm. Biomed. Anal., 1994, vol. 12, no. 9, p. 1109-1113.
Teixeira, M.F.S., Marcolino-Júnior, L.H., Fatibello-Filho, O., Dockal, E.R., Cavalheiro, E.T.G. Voltammetric determination of dipyrone using a N,N’-ethylenebis(salicylideneaminato)oxovanadium(IV) modified carbon-paste electrode. J. Braz. Chem Soc., 2004, vol. 15, no. 6, p. 803-808.
Senyuva, H.Z., Aksahin, I., Ozcan, S., Kabasakal, B.V. Rapid, simple and accurate liquid chromatography–diode array detection validated method for the determination of dipyrone in solid and liquid dosage forms. Anal. Chim. Acta., 2005, vol. 547, no. 1, p. 73-77.
Loginova, L.P., Konovalova, O.Yu. Test films for test-determinations on the base of reagents, immobilized in gelatinous gel. Talanta, 2008, vol. 77, no. 2, p. 915-923.
Ruzicka, J. Flow injection analysis. 2nd Ed., N.Y.: J. Wiley, 1988, 498 p.
Ruzicka, J., Marshall, G.D. Sequential injection: a new concept for chemical sensors, process analysis and laboratory assays. Anal. Chim. Acta., 1990, vol. 237, p. 329-343.
Daniel, D., Gutz, I.G.R. Electronic micropipettor: A versatile fluid propulsion and injection device for micro-flow analysis. Anal. Chim. Acta., 2006, vol. 571, no. 2, p. 218-227.
Huang, Y.-M., Zhang, C., Zhang, X.-R., Zhang, Z.-J. A novel chemiluminescence flow-through sensor for the determination of analgin. Fres. J. Anal. Chem., 1999, vol. 365, no. 4, p. 381-383.
Calatayud, J.M. Flow Injection Analysis of Pharmaceuticals, London: Taylor & Francis, 1996, 315 p.
Albuquerque, J.S., Silva, V.L., Araújo, A.N., Montenegro, M.S.B.S.M. Determination of dipyrone in pharmaceutical products by flow injection analysis with potentiometric detection. Anal. Sci., 2003, vol. 19, no. 5, p. 691-694.
Boni, A.C., Wong, A., Dutra, R.A.F., Sotomayor, M.D.P.T. Cobalt phthalocyanine as a biomimetic catalyst in the amperometric quantification of dipyrone using FIA. Talanta, 2011, vol. 85, no. 4, p. 2067-2073.
Weinert, P.L., Fernandes, J.R., Pezza, L., Pezza, H.R. Flow-Injection Spectrophotometric Determination of Novalgin in Pharmaceuticals Using Micellar Medium. Anal. Sci., 2007, vol. 23, no. 12, p. 1383-1389.
Petrushina, G.A., Tsiganok, L.P., Vishnikin, A.B. Spectrophotometric determination of ascorbic acid by P2Mo18O626-. Ukr. Khim. Zhurn., 2010, vol. 36, no. 4, p. 116-122.
Bulatov, A.V., Petrova, A.V., Vishnikin, A.B., Moskvin, A.L., Moskvin, L.N. Stepwise injection spectrophotometric determination of epinephrine. Talanta, 2012, vol. 96, p. 62-67.
Vishnikin, A.B., Sklenařova, H., Solich, P., Petrushina, G.A., Tsiganok, L.P. Determination of ascorbic acid with Wells-Dawson type molybdophosphate in sequential injection system. Anal. Lett., 2011, vol. 44, no. 1-3, p. 514-527.
Vishnikin, A.B., Al-Shwaiyat, M.K.E.A., Petrushina, G.A., Tsiganok, L.P., Andruch, V., Bazel, Ya.R., Sklenarova, H., Solich, P. Highly sensitive sequential injection determination of p-aminophenol in paracetamol formulations with 18-molybdodiphosphate heteropoly anion based on elimination of Schlieren effect. Talanta, 2012, vol. 96, p. 230-235.
Saeed, A. Spectrophotometric determination of novalgin in pharmaceutical preparations by the molybdenum blue method. Indian J. Pharm. Sci., 1998, vol. 60, no. 3, p. 161-163.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

