Tautomerism of 4-hydrazinoquinazolines: vibrational spectra and computational study
DOI:
https://doi.org/10.15421/081303Keywords:
tautomer, 4-hydrazinoquinazolines, ab-initio, IR spectra, vibrational assignmentAbstract
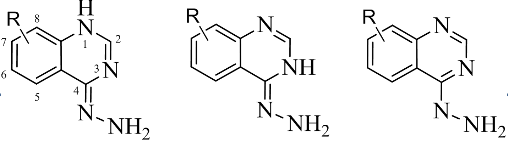
The tautomerism of 4-hydrazinoquinazoline and its derivatives was investigated. Geometry and thermodynamic parameters were computed theoretically using Gaussian 03 software. All calculations were performed at the MP2 level of theory using the standard 6-31G(d) basis. Energetics and relative stabilities of tautomers were compared and analyzed in a gas phase. The effect of solvents (1,4-dioxane, acetic acid, ethanol and water) on the tautomeric equlibria was evaluated using PCM. It was determined that solvents induced slight changes in the relative stability. In all cases 4-hydrazinoquinazoline exists predominantly as the amino form. The variation of dipole moments was studied. The anharmonic vibrational wavenumbers for unsubstituted 4-hydrazinoquinazoline were calculated at MP2/6-31G(d) level and compared with experimental data. The modes of IR spectra were assigned. The calculated herein wavenumbers and intensities of amino form are in good agreement with those observed experimentally.References
Karpenko, O. V., Kovalenko, S. I., Chekotylo, O. O., Shishkina, S. V. A new one-step synthesis of 1,2,4-triazino[2,3-c]quinazolines. Heterocycles, 2007, vol. 71, no. 3, p. 619-626.
Hariharan, P. C., Pople, J. A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta., 1973, vol. 28, no. 3, p. 213-222.
Moller, C., Plesset, M. S. Note on an approximation treatment for many-electron systems. Phys. Rev., 1934, vol. 46, p. 618-622.
GaussView 3.0, Gaussian Inc., Carnegie Office, Park, Pittsburgh, PA 15106, USA.
Gorelsky, S. I. SWizard Program, Revision 2.0, York University: Ontario, 2001, http://www.obbligato.com/software/swizard/
Mohamed, A. A., El-Harby, A. W. Substituent effect on the amino-imino tautomerism of aminothiazoles. Journal of Molecular Structure: Theochem, 2008, vol. 849, no. 1, p. 52-61.
Jalilian, M. R., Zahedi-Tabrizi, M. The most stable tautomer of 3-amino-1,2,4-triazin-5-one and its structural geometry. Spectrochimica Acta Part A, 2008, vol. 70, no. 5, p. 1020-1024.
Afifi, M. S., Farag, R. S., Shaaban, I. A., Wilson, L. D., Zoghaib, W. M., Mohamed, T. A. Infrared and NMR spectra, tautomerism, vibrational assignment, normal coordinate analysis, and quantum mechanical calculations of 4-amino-5-pyrimidinecarbonitrile. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2013, vol. 111, p. 277-289.
Srivastava Rajesh, K., Narayan, V., Kumar, A., Prasad, O. Theoretical Studies on the Isomers of Quinazolinone by first Principles. Research Journal of Recent Sciences, 2012, vol. 1, no. 3, p. 11-18.
Prasad, O., Sinha, L., Kumar, N. Theoretical Raman and IR spectra of tegafur and comparison of molecular electrostatic potential surfaces, polarizability and hyperpolarizability of tegafur with 5-fluoro-uracil by density functional theory. J. At. Mol. Sci., 2010, vol. 1, p. 201-214.
Jahubar Ali, A., Thangarasu, S., Athimoolam, S., Asath Bahadur S. Experimental And Theoretical Vibrational Spectra, Factor Group Analysis And Quantum Chemical Calculations Of Creatininium Benzoate. International Journal of ChemTech Research, 2013, vol. 5, no 4, p. 1694-1706.
Panicker, C. Y., Varghese, H. T., Ambujakshan, K. R., Mathew, S., Ganguli, S., Nanda, A. K., Alsenoy, C. V., Mary, Y. S. Vibrational spectra and computational study of 3-amino-2-phenyl quinazolin-4(3H)-one. Journal of Molecular Structure, 2010, vol. 963, p. 137-144.
Coates, J., Meyers, R. A. Introduction to Infrared Spectrum, A Practical Approach, Chichester: John Wiley and Sons Ltd., 2000, p. 23.
Roeges, N. P. G. A Guide to the Complete Interpretation of Infrared Spectra of Organic Compounds, New York: Wiley, 1994, p. 356.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

