Reactions of 2,3-dihydro-1,5-benzodiazepinones-2 derivatives quaternization
DOI:
https://doi.org/10.15421/081304Keywords:
1, 5-dihydrobenzodiazepinone-2, alkylation, quaternizationAbstract
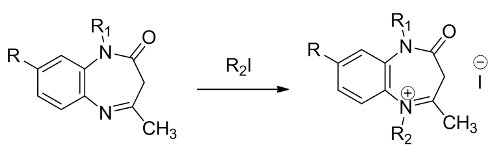
Alkylation of 1-alkyl-4-methyl-2,3-dihydro-1,5-benzodiazepinones-2 with alkyl halides is shown to result in the formation of quaternary salts. In contrast to 1,5-benzodiazepinones-2, unsubstituted at position 1, quaternization of 1-alkyl-derivatives requires larger excess of alkyl halide and prolonged (18-46 h) boiling in benzene. Quaternary salts are obtained in yields 45-77% as crystalline deposits or oils which crystallized upon grinding with ether or hexane. The yields depended on the nature of alkyl halide and the substituent at position 4. The yields obtained with ethyl iodide were notably lower compared to those with methyl iodide, and the reactions were significantly slower. At the same time, quaternization of 1-alkyl-4-phenyl-2,3-dihydro-1,5-benzodiazepinones-2 did not proceed even upon boiling in benzene with methyl iodide for 80 h.References
Andronati, S. A. Meditsinskaya himiya. Struktura, svoystva, molekulyarnyie mehanizmyi deystviya biologicheski aktivnyih veschestv, Odessa: Astroprint, 2006, 132 p.
Bogatskiy, A.V., Andronati, S.A., Golovenko, N.Ya. Trankvilizatoryi. 1,4-Benzodiazepinyi i rodstvennyie strukturyi, Kiev, 1980, 280 p.
Gaponov, A. A. Reaction of 2,3-dihydro-1H-1,5-2-benzodiazepinonov with alkylating agents. Visn. Dnipropetr. Univ.: Khim., 2010, no. 16, p. 99-104.
Vernin, D., Domloi, H., Siv, C., Metzger, J., Archavlis, A., Llinas, J. R. Alkylation en catalyse par transfert de phase de dihydro-1,3-(2H)benzo[2,3-b]diazepines-1,5-ones-2. Chem. Scripta, 1980, no. 16, p. 157-162.
Bellami, L. Infrakrasnyie spectryi slognuch molekul, Moscow: IL, 1963, 592 p.
Nakanisi, K. Infrakrasnyie spectryi i stroenie organicheskich soedinenij, Moscow: Mir, 1965, 216 p.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

