N-chloro-N-benzoiloxy benzamide
DOI:
https://doi.org/10.15421/081307Keywords:
N-chloro-N-acyloxybenzamides, N-acyloxybenzamides, nucleophilic substitution at nitrogen atomAbstract
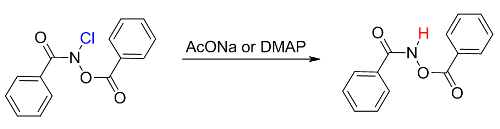
N-Chloro-N-(benzoyloxy)benzamide has been synthesized by chlorination of N-benzoyloxybenzamide with tert-butylhypochlorite. The structure of N-chloro-N-(benzoyloxy)benzamide has been соnfirmed by its NMR 1H and mass-spectra. Upon storage at −26 °C, the labile N-chloro-N-(benzoyloxy)benzamide quickly converts into N-(benzoyloxy)benzamide. Reaction of N-chloro-N-(benzoyloxy)benzamide with anhydrous sodium acetate in acetonitrile gives N-benzoyloxybenzamide. 4-N,N-Dimethylaminopyridine reacts with N-chloro-N-(benzoyloxy)benzamide in acetonitrile leading to 4-N,N-dimethylaminopyridine hydrochloride and N-benzoyloxybenzamide. In both cases, the products of nucleophilic substitution at the amide nitrogen have not been found. The N-Cl bond polarization in N-chloro-N-(benzoyloxy)benzamide disfavors nucleophilic substitution at the amide nitrogen. Investigation of the interactions of N-chloro-N-(benzoyloxy)benzamide with AcONa and 4-N,N-dimethylaminopyridine has shown that nucleophilic substitution at the nitrogen atom does not occur in the case of N-chloro-N-acyloxyamides.References
Shtambyrg, V. G., Pleshkova, A. P., Serdyk, V. N., Ivonin, S. P. N-Acetoxy-N-methoxyurethilan. J. Org. Chem., 1999, no. 7, p. 1120. Shtambyrg, V. G., Pleshkova, A. P., Serdyk, V. N., Ivonin, S. P. N-Acyloxy-N-alkoxyureas. J. Org. Chem., 1999, no. 10, p. 1578-1579. Shtambyrg, V. G., Klots, E. A., Pleshkova, A. P. Geminal system. Message 50. Synthesis and alcoholysis N-alkoxy-N-acyloxy derivatives of ureas, carbamates and substituted benzamides. Izv. RАN.: Khim, 2003, no.10, p. 70-73. Shtambyrg, V. G., Кravchenko, S. V., Olefir, D. A. "Deformed" ureas. Visn. Dnipropetr. Univ. : Khim, 2007, no. 13, p. 85-97. Shtambyrg, V. G., Klots, E. A., Serdyk, V. N., Pleshkova, A. P. Nucleophilic substitution on the nitrogen atom in the N-alkoxy-N-chlorocarbabames, N-alkoxy-N- acyloxycarbabames and N-alkoxy-N-acyloxyureas. Visn. Dnipropetr. Univ. : Khim, 2000, no. 5, p. 13-27. Shtambyrg, V. G., Klots, E. A., Serdyk, V. N. Preparation and alcoholism N-alkoxy-N-acyloxyureas. Ukr. Кhim. Jurn., 2002, no. 7, p. 49-55. Shtambyrg, V. G., Kravchenko, S. V., Tsygankov, A. V. N-Chloro-N-alkoxyureas as a new kind of "anomeric" amides. Influence of the nature N'-substituents on the reactivity of N-chloro-N-alkoxyaminogroup. Visn. Dnipropetr. Univ.: Khim, 2006, no. 12, p. 68-76. Shtambyrg, V. G., Rudchenko, V. F., Nasibov, Sh. S. N-Chloro-N-methoxyurethilan. Izv. АN. USSR.: Khim, 1981, no. 2, p. 449-452. Glover, S. A. Anomeric Amides – Structure, Properties and Reactivity. Tetrahedron, 1998, Vol. 54, no. 26, p. 7229-7271.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

