The influence of temperature on manganese oxides electrodeposition on platinum and steel electrodes
DOI:
https://doi.org/10.15421/081309Keywords:
manganese oxide, the influence of temperature, substrate material, the mechanism of precipitate formationAbstract
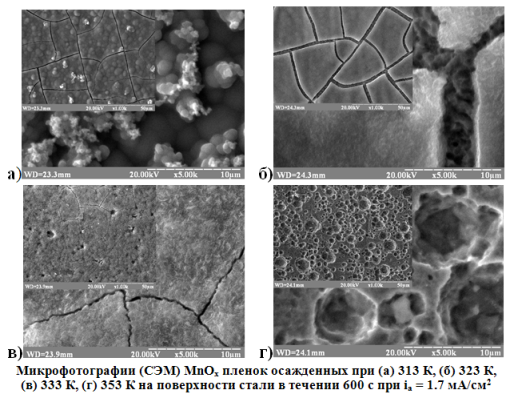
The influences of temperature and substrate material (platinum, steel 12С18N10T) on the mechanism of the electrochemical oxidation of Mn2+ to MnOx in the acetic acid electrolyte were investigated using hronovoltamperometry method. Analysis of polarization curves at T ≤ 323 K proved that the hydrolysis reaction like rate-determining step strongly influenced the process of oxide film formation. It was observed the additional oxidation peak currents on i, E - curves at T ≥ 323 K. It has been interpreted like another manganese oxide film formation mechanism take place. It was experimentally found that the substrate material didn’t substantial effect on the MnOx electrodeposition process. The structures of films formed at T ≥ 333 K was investigated using X-ray diffraction. The polycrystalline coatings consisting of a mixture γ - MnO2 and Mn3O4 phases with different crystallographic orientation were observed. The analysis of XRD patterns was allowed to estimate the dependence of Mn3+ / Mn4+ratio in the film from the temperature of coating deposition. It was determined the dependence of the crystalline degree from the temperature of coating deposition. Scanning electron microscopy (SEM) was used to study the morphology of the obtained samples. It was found a significant cracking on the coating obtained at T = 323 K. It has been explained changing of hydration degree in the oxide.References
Zaretskiy, S. A., Suchkov, V. N., Shlyapnikov, V. A. Tehnologiya elektrohimicheskih proizvods, Moskow, 1970, 423 р.
Duarte, M.M.E., Pilla, A.S., Mayer, C.E. Electrooxidation of Mn(II) to MnO2 on graphite fibre electrodes. J. Appl. Electrochem., 2003, vol. 33, p. 387-392.
Petitrierre, J-Ph., Comninellis, Ch., Plattner, E. Oxydation Du MnSO4 en dioxide de manganese dans H2SO4 30%. Electrochem. Acta, 1990, vol. 35, no. 1, p. 281 287.
Davies, G. Coord. Some aspects of the chemistry of manganese (III) in aqueous solution. Chem. Rev., 1969, vol. 4, p. 199-224.
Rogulski, Z., Chotkowski, M. Electrochemical behavior of MnO2/RVC system. Journal of New Materials for Electrochemical Systems, 2006, no. 9, p. 401-408.
Gorbachev, S.V. Effect of temperature on electrolysis as kinetic method for investigating the nature of the electrochemical processes. Chetvertoe soveschanie po elektrohimii. Sb. nauchnyih trudov, 1959, p. 61-71.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

