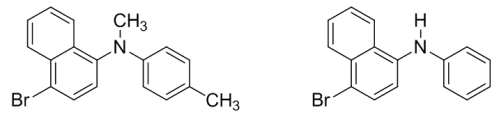
1H NMR spectra of N-methyl-4-tolyl-1-(4-bromonaphthyl)amine and N-phenyl-1-(4-bromonaphthyl)amine: a combined experimental and theoretical study
DOI:
https://doi.org/10.15421/081313Keywords:
N-methyl-4-tolyl-1-(4-bromonaphthyl)amine, N-phenyl-1-(4-bromonaphthyl)amine, NMR, DFT, basis setAbstract
Theoretical investigations of the conformational properties and 1H NMR chemical shifts for N-methyl-4-tolyl-1-(4-bromonaphthyl)amine and N-phenyl-1-(4-bromonaphthyl)amine are reported. The calculations were performed at the DFT level (PBE1PBE functional) using magnetically consistent 6-31G## and STO##-3Gmag basis sets. Conformational properties of the amines were studied using potential energy surface scanning. Chemical shifts were calculated using the GIAO and CSGT methods and averaged in proportion to the population of the corresponding conformations. Solvent effects (CDCl3) were accounted via PCM method. The obtained results allowed to assign the 1H NMR signals for the naphthalene moiety, which could not be done based on the experimental data alone.References
Beilstein Handbuch der organishen Chemie. Berlin, Bd. 5, p. 1224-1226.
Buu-Hoi, N.P., Royer, R., Hubert-Habart, M. Carcinogenic Nitrogen Compounds. Part XVII. The Synthesis of Angular Benzacridines. J. Chem. Soc., 1955, p. 1082-1084.
Ivonin, S. P., Kopteva, S. D., Serdyuk, V. N. Phosphorilation of diarylamines. Zhurn. organ. khim., 2000, vol. 36, no. 3, p.422-428.
Ivonin, S. P., Kopteva, S. D., Dmitrikova, L.V. Phosphorilation of N methyldiarylamines. Visn. Dnipropetr. Univ.: Khim, 2002, no. 8, p. 57-62.
Ivonin, S. P., Kopteva, S. D., Dmitrikova, L. V., Glushko, A. I. N-methyl-4-tolyl-1-naphtylamin in electrophilic substitution reactions. Visn. Dnipropetr. Univ.: Khim, 2004, no. 10, p. 40-47.
Kopteva, S. D., Dmitrikova, L.V., Glushko, A. I. Phenyl-1-naphthylamin in electrophilic substitution reactions. Visn. Dnipropetr. Univ.: Khim, 2005, vol. 11, no. 7, p. 93-95.
Kopteva, S. D., Dmitrikova, L.V., Dunyashenko, Ya.A. Selective bromination of arylnaphthylamines. Visn. Dnipropetr. Univ.: Khim, 2007, vol.13, no. 10/2, p. 131 136.
Kopteva, S. D., Dmitrikova, L.V., Velichenko, Yu.O. Formilation of diarylamines and their N-methyl derivatives in the conditions of Haak reaction. Visn. Dnipropetr. Univ.: Khim, 2010, no. 16, p. 93-99.
Okovytyy, S., Kasyan, L., Seferova, M., Rossikhin, V., Svjatenko, L., Leszczynski, J. Identification of the Stereoisomers of Tetrahydroindene Diepoxide by the 1H and 13C NMR Characteristics: A Combined Experimental and Theoretical Study. J. Mol. Struct.: Theochem., 2005, vol. 730, no 1-3, p.125-132.
Rossikhin, V. V., Kuz'menko, V. V., Voronkov, E. O., Zaslavskaya, L. I. Improvement of STO and GTO Basis Set Quality in Calculations of Magnetic Properties by the Coupled and Uncoupled Hartree-Fock Perturbation Theory. J. Phys. Chem., 1996, vol. 100, no. 51, p. 19801-19807.
Bolotin A., Rossikhin V., Voronkov E. Ab initio calculations of polarizabilities of small molecules with Gauss-type function depending on perturbation. Acta Phys. Hung., 1991, vol 70, no. 4, p. 299-316.
Rossikhin, V. V., Okovytyy, S., Kasyan, L. I., Voronkov, E. O., Umrikhina, L. K., Leszczynski, J. An Investigation of the 17O NMR Chemical Shifts in Oxiranes Using Magnetically Corrected Basis Sets. J. Phys. Chem. A, 2002., vol. 106, no. 16, p. 4176-4180.
Bolshakov, V., Rossikhin, V., Voronkov, E., Okovytyy, S., Leszczynski, J. Accurate Calculations of Second-Order Electric and Magnetic Properties: Two Ways of Physically Justified Modifications of Basis Sets. Chem. Phys., 2010, vol. 372, no. 1-3, p. 67-71.
Okovytyy, S., Umrikhina, L. K., Isaev, O. K. Quantum-chemical investigation of stereoisomeric epoxinorbornanes 1H and 13C NMR spectra parameters. Visn. Dnipropetr. Univ.: Khim., 2001, no. 6, p. 50-54.
Sviatenko, L. K., Okovytyy, S., Kasyan, L.I. Chemical shifts of 13C and 1H nuclei in NMR spectra of olefins. Visn. Dnipropetr. Univ.: Khim., 2005, no. 11, p. 70-75.
Bolshakov, V., Rossikhin, V., Voronkov, E., Okovytyy, S., Leszczynski, J. The performance of the 6-31G## basis set and its modifications for DFT calculation of nuclear magnetic shielding and spin-spin coupling constants. Sci. Israel–Tech. Adv., 2008, no. 10, p. 120.
Okovytyy, S. I., Voronkov, E. O., Rossikhin, V. V., Balalayev, O. K., Leszczynski, J. New Approach for Calculations of the Second-Order Magnetic Properties: Magnetic Susceptibility. J. Phys. Chem. A., 2004, vol. 108, no. 22, p. 4930 4933.
Voronkov, E., Rossikhin, V., Okovytyy, S., Shatckih, A., Bolshakov, V., Leszczynski, J. Novel physically adapted STO##-3G basis sets. Efficiency for prediction of second-order electric and magnetic properties of aromatic hydrocarbons. Int. J. Quant. Chem., 2012, vol. 112, no. 12, p. 2444-2449.
Bolshakov, V. I., Rossikhin, V. V., Okovуtyу, S. I., Voronkov, E.O., Leszczynski, J. The performance of the new 6-31G## basis set: Molecular structures and vibrational frequencies of transition metal carbonyls. J. Comput. Chem., 2007, vol. 28, no. 4, p. 778-782.
Keith, T. A., Bader, R. F. W. Calculation of magnetic response properties using atoms in molecules. Chem. Phys. Lett., 1992, vol. 194, no. 1, p. 1-8.
Ditchfield, R. On molecular orbital theories of NMR chemical shifts. Chem. Phys. Lett., 1972, vol. 15, no. 2, p. 203-206.
Wolinksi, K., Hinton, J. F., Pulay, P. J. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc., 1990, vol. 112, no. 23, p. 8251-8260.
Frisch, M. J., Trucks, G. W., Schlegel, H. B. et al. Gaussian 09, Revision A.02, Gaussian, Inc.: Wallingford CT, 2009.
Miertus, S., Scrocco, E., Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys., 1981, vol. 55, no. 1, p. 117-129.
Pretsch, E., Bühlmann, P., Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data. Springer-Verlag Berlin Heidelberg, 2009, 452 p.
Abraham, R. J., Mobli, M., Smith, R. J. 1H chemical shifts in NMR. Part 20.– Anisotropical steric effects in halogen substituent chemical shifts (SCS), a modeling and ab initio investigation. Magn. Reson. Chem., 2004, no. 42, p. 436-444.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

