New oxazilines with sulfolane frame
DOI:
https://doi.org/10.15421/081315Keywords:
sulfolane, aminotetragidrotiofen-4-3-ol-1, 1-dioxide, the aminoalcohol, 1, 3-oxazolin, amideAbstract
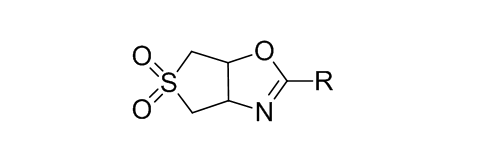
Oxazolines are widely used as synthons for medicines and their production, as protection for structural fragments of reaction centers, as well as ligands. We for the first time examined the ability of cis- and trans-3-hydroxy-4-aminosulfolanov to form oxazolines. Oxazolines are formed by cyclization of the corresponding N-acyl derivatives of trans-3-hydroxy-4-aminosulfolan under reflux in thionyl chloride; amides of cis-isomer aminoalcohol under the same conditions of the reaction do not formed identically product. These results confirm that the oxazoline cycle formed by realization of intramolecular SN2- mechanism using thionyl chloride as dehydrating agent; alternative mechanism (the attack of the hydroxyl group on carbon of amide group) can not be realized using the microwave radiation reaction mass.References
Inaba, T., Yamada, Y. US Patent no. 6476232, 2002.
Tao, J., Zhao, L., Ran, N. Recent Advances in Developing Chemoenzymatic Processes for Active Pharmaceutical Ingredients. Org. Proc. Res. Dev, 2007, no. 2, p. 259-267.
Lait, S. M., Rankic, D. A., Keay, B. A. 1,3-Aminoalcohols and their derivatives in asymmetric organic synthesis. Chem. Rev., 2007, vol. 107, no. 3, p. 767-796.
Ito, J., Nishiyama, H. Synthetic utility of chiral bis(oxazolinyl)phenyl transition-metal complexes/ J. Ito, H. Nishiyama. Synlett, 2012, no. 23, p. 509-523.
Cossu, S., Giacomelli, G., Conti, S., Falorni, M. Unusual reactivity of 4-carboxyamido-2-oxazoline systems: new synthesis of optically active n-sulphonyl derivatives. Tetrahedron, 1994, vol. 50, no. 17, p. 5083-5090.
Pham, V. T., Joo, J.-E., Tian, Y.-S., Chung, Y.-S., Lee, K.-Y., Oh, C.-Y., Ham, W.-H. Stereoselective total synthesis of (2S,3R)-3-hydroxypipecolic acid. Tetrahedron: Asymmetry, 2008, vol. 19, no. 3, p. 318-321.
Gwaltney, S. L., Jae, H.-S., Kalvin, D. M., Liu, G., Sham, H. L., Li, Q., Claiborne, A. K., Wang, L., Barr, K. J., Woods, K. W. US Patent 6228868, 2001.
Kasyan, L. I., Palchikov, V. A., Tokar, A. V. Oksazageterotsiklyi na osnove aminospirtov, epoksidov i aziridinov, Dnipropetrovsk: Izd-vo DNU, 2012, 644 p.
Ciufolini, M. A. Synthetic studies on heterocyclic natural products. Il Farmaco, 2005, vol. 60, no. 8 , p. 627-641.
Ager, D. J., Prakash, I., Schaad, D. R. 1,2-Amino Alcohols and Their Heterocyclic Derivatives as Chiral Auxiliaries in Asymmetric Synthesis Chem. Rev, 1996, vol. 96, no. 2, p. 835-875.
Kasyan, L. I., Palchikov, V. A., Bondarenko, Ya. S. Five-Membered Oxaza Heterocyclic Compounds on the Basis of Epoxides and Aziridines. Russian Zhurn. Org. Khimii, 2011, vol. 47, no. 6, р. 791-829.
Yamada, N., Mizuochi, M., Takeda, M., Kawaguchi, H., Morita H. A facile and efficient one-pot synthesis of thiirans by the reaction of benzoxazolyl β-ketosulfides with NaBH4/NaOH. Tetrahedron Lett, 2008, vol. 49, no. 7, p. 1166-1168.
Bezmenova, T. E., Dulnev, P. G., Malyuk, L. G., Rudzit, E. A., Kulikova, I. A. USSR Patent no. 745161, 1986.
Tolstikov, G. A., Novitskaya, N. N., Flekhter, B. V., Lazareva, D. N., Davydova, V. A., Kamalova É. G. Derivatives of sulfolane, a new class of antiinflammatory compounds. Pharm. Chem. J., 1978, vol. 12, no. 12, p. 33-38.
Zlenko, O. T., Mamchur, V. Y., Kas’yan, L. I., Palchikov, V. O., Prishlyak, I. S., Dulnev, P. G., Tarabara, I. M., Stefanik, M. I. UA Patent 69026, 2012.
Zlenko, O. T., Mamchur, V. Y., Palchikov, V. O., Zarovna, I. S., Dulnev, P. G., Shastun, N. P., Ivanov, A. V. UA Patent 74602, 2012.
Dulnev, P. G., Bezmenova, T. E. USSR Patent no. 562088, 1985.
Suhoveev, V. V., Tsigankov, S. A., Shvidko, O. V. Sulpholane containing amino acids: synthesis and biological effect. Tezi dop. XIX Vseukr. konf. z org. khimii, Lviv, 2001, p. 289.
Schwekendiek, K., Glorius, F. Efficient Oxidative Synthesis of 2-Oxazolines. Synthesis, 2006, no. 18, p. 2996-3002.
Sorenson, W. R. Epoxidation of butadiene sulfone. J. Org. Chem., 1959, vol. 24, no. 11, p. 1796-1798.
Zarovnaya, I. S., Tokar, A. V., Palchikov, V. A. Investigation of the mechanism of aminolysis of 3,4-epoxysulfolane-1,1-dioxide. X Vseukrayinska konferentsiya molodih vchenih ta studentiv z aktualnih pitan himiyi, Tezi dopovidey, Dnipropetrovsk, 2012, p. 14.
Chou, T., Chen, H.-C., Tsai, C.-Y. Preparation of (phenyloxazolo)-3-sulfolene. A precursor for (phenyloxazolo)-o-quinodimethane. J. Org. Chem., 1994, vol. 59, no. 8, p. 2241-2245.
Lutz, R. E., Wayland, R. L. Further Studies on the Stability of β-Hydroxyethylamines toward the Oppenauer Oxidation. Cis and trans-1-Amino-2-indanols. J. Am. Chem. Soc., 1951, vol. 73, no. 4, p. 1639-1641.
Thompson, W. J., Fitzgerald, P. M. D., Holloway, M. K., Emini, E. A., Darke, P. L., McKeever, B. M., Schleif, W. A., Quintero, J. C., Zugay, J. A. Synthesis and antiviral activity of a series of HIV-1 protease inhibitors with functionality tethered to the P1 or P1' phenyl design. J. Med. Chem., 1992, vol. 35, no. 10, p. 1685-1701.
Myllymäki, M. J., Koskinen, A. M. P. A rapid method for the preparation of 2-substituted oxazolo[4,5-b]pyridines using microwave-assisted direct condensation reactions.Tetrahedron Lett., 2007, vol.48, no. 13, p. 2295-2298.
Ishihara, M., Togo, H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine.Tetrahedron, 2007, vol.63, no. 6, p. 1474-1480.
Yang, Y.-H., Shi, M. Selective syntheses of benzoxazoles and N-(2-hydroxyaryl) pyrrolidin-2-ones from the corresponding cyclopropyl amines with PPh3/CX4. Tetrahedron, 2006, vol. 62, p. 2420-2427.
Hammam, A. S., Yanni, A. S., Khalil, Z. H. Synthesis of some new ozazoloquinolines and stilbyloxazoloquinolines. J. Chem. Tech. Biotechnol., 1982, no. 32, p. 485-488.
Somanathan, R., Aguilar, H. R., Rivero, I. A., Aguirre, G., Hellberg, L. H.; Yu, Z., Thomas, J. A. Thermal cyclisation of β-hydroxyamides to oxazolines. J. Chem. Res. Synop., 2001, no. 3, p. 92-92.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

