Unusual spontaneous α→β isomerization of unsymmetrical benzoins. Products and their structure
DOI:
https://doi.org/10.15421/081316Keywords:
benzoins, arylglyoxals, isomerizationAbstract
The article describes reactions of a series of arylglyoxals with 2-methylfuran and
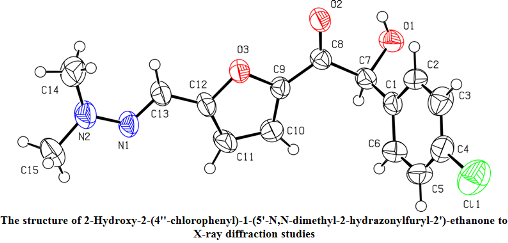
furfural N,N-dimethylhydrazone. These interactions lead selectively to unsymmetrical benzoins. It was found that some of the benzoins underwent spontaneous thermal α→β benzoin isomerization in situ. The rearrangement took place in the absence of bases, which could be explained by two structural factors: (a) the presence of a halogen atom in the para-position of the aryl moiety, and (b) the presence of the Me2NN=CH-substituent in the 5-position of the furan ring. The proposed mechanism of the thermal rearrangement starts with an intramolecular protonation of the carbonyl oxygen by the hydroxyl. This leads to the 1,2-hydride shift onto the carbonyl group, finally yielding β-benzoins. The S-isomer of 2-hydroxy-2-(4’’-chlorophenyl)-1-(5’-N,N-dimethylhydrazonylfuryl-2’)-ethanone-1 was isolated by crystallization, and its structure was confirmed by the X-ray crystallography.
References
Ivonin, S. P., Anishchenko, A. A., Samucha, A. V., Lapandin, А. V., Serduk, V. N., Pleshkova, A. P., Shtamburg, V. G. Arylfuraciloines. Visn. Dnipropetr. Univ.: Khim., 2000, no. 5, p. 27-32.
Ivonin, S. P., Lapandin, A. V., Anishchenko, A. A., Shtamburg, V. G. Reaction of Arylglyoxales with Electron-Rich Benzenes and pi-Excessive Heterocycles. Facile synthesis of Heteroaryl alpha-Aciloins. Synth. Commun., 2004, vol. 34, p. 451-461.
Ivonin, S. P., Lapandin, A. V., Anishchenko, A. A., Shtamburg, V. G. Mutual Influence of (Dimethylhydrazono)methyl group and alpha-Hydroxy Ketone Molecules in Hetaryl Analogues of unsymmetric Benzoines. Eur. J. Org. Chem., 2004, p. 4688-4693.
Buck, J. S., Ide, W. S. The Synthesis of Benzoins. Organic Reactions., Wiley : New York, 1949, vol. 4, p. 269-304.
Julian, P. L., Passler, W. The Thermal Interconversion of Mixed Benzoines. J. Am. Chem. Soc., 1932, vol. 54, p. 4756.
Sheldrick, G. Short history of SHELX. Acta Cryst., Sect. A, 2008, vol. 64, p. 112.
Van der Sluis, P., Spek, A. L. BYPASS: an effective method for the refinement of crystal structures containing disordered solvent regions. Acta Cryst., Sect A, 1990, vol. 46, p. 194-201.
Spek, A. L. J. Single-crystal structure validation with the program PLATON. Appl. Cryst., 2003, vol. 36, p. 7-13.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

