Chemical properties of 2,3-dihydro-1H-1,5-benzodiazepinone-2 derivatives – a review
DOI:
https://doi.org/10.15421/081319Keywords:
1, 5-dihydrobenzodiazepinone-2, biological activity, reaction of electrophilic substitution, reaction with nucleophilic reagentsAbstract
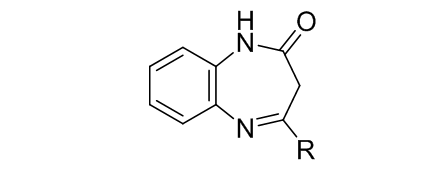
Condensed heterocyclic systems with two nitrogen atoms in the seven-membered ring – benzodiazepines and their derivatives – are interesting and important objects from both theoretical and applied point of view. They attract a lot of attention due to their potent and diverse biological activities. These compounds include numerous tranquilizers, sedatives, analgesics, as well as anticonvulsant, anti-inflammatory and anti-tumor agents. The presence of several reactive centers in 1,5-benzodiazepines leads to a variety of reactions with both electrophilic and nucleophilic agents. The first part of this review covers electrophilic substitution of 2,3-dihydro-1H-1,5-benzodiazepin-2-ones (nitration, halogenation, alkylation, acylation, formylation, etc.) The data shows that the reaction outcome is determined by the nature of both the substrate and the electrophilic agent, as well as by the reaction conditions: temperature, time, and solvent.
References
Solomko, Z. F., Kost, A. N. 1,5-Benzodiazepines (review). Chem. Heterocycl. Compd., 1975, no. 11, р. 1231-1248.
Bogatskiy, A. V., Andronati, S. A., Golovenko, N. Ya. Trankvilizatoryi. 1,4-Benzodiazepinyi i rodstvennyie strukturyi, Kiev, 1980, 280 p.
Gaponov, A. A. Synthesis and biological activity of 2,3-dihydro-1H-1,5-benzodiazepin-2-ones. Visn. Dnipropetr. Univ.: Khim., 2012, no. 18, p. 82-90.
Puodzhyunaite, B. A., Talaikite, Z. A. Synthesis of nitro-substituted 4-methyl-2,3-dihydro- and 2,3,4,5-tetrahydro-1H-1,5-benzo-2-diazepinones. Chem. Heterocycl. Compd., 1974, no. 6, р. 724-727.
Chmilenko, T. S. Sintez i nitrovanie dihidro-1,5-benzodiazepinonov-2. PhD dissertation, Dnipropetrovsk, 1984.
Solomko, Z. F., Chmilenko, T. S., Sharbatyan, P. A., Shevchenko, L. V., Bozhanova, N. Ya. Nitration of 8-substituted 2,3-dihydro-1H-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1978, no. 4, р. 455-458.
Solomko, Z. F., Chmilenko, T. S., Sharbatyan, P. A., Shtemenko, N. I., Khimyuket, S. I. Nitration of 7- and 8-methyl-4-R-1H-2, 3-dihydro-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1978, no. 1, р. 100-104.
Chmilenko, T. S., Solomko, Z. F., Kost, A. N. Synthesis and nitration of 4-phenyl-2,3-dihydro-1H-1,5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1977, no. 4, р. 423-427.
Solomko, Z. F., Chmilenko, T. S., Schevchenko, L. V., Chimyuk, S. I. 2,3-Dihydro-1,5-benzodiazepinones in electrophilic substitution reaction. Dnipropetrovsk, 1977. – Dep. VINITI 08.06.77 № 2309-77. 9 p.
Solomko, Z. F., Avramenko, V. I., Pribega, L. V. Bromo derivatives of 4-phenyl-2,3-dihydro-1h-1,5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1978, no. 3, р. 340-344.
Sobol', L. V., Solomko, Z. I., Avramenko, V. I., Khmel', M. P. Bromination of 8R-4-phenyl-2,3-dihydro-1H-1,5-benzodiazepinones-2. Chem. Heterocycl. Compd., 1984, no. 8, р. 927-930.
Solomko, Z. F., Sheremet, V. I., Khmel', M. P., Avramenko, V. I., Proshkina V. N. Chloro derivatives of 4-phenyl-2,3-dihydro-1H-1,5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1982, no. 3, р. 307-310.
Scheremet, V. I. Chlorproizvodnye arilaminokrotonatov i 1,5-benzodiazepinonov-2. PhD dissertation, Dnipropetrovsk, 1982.
Avramenko, V. I., Scharbatyan, P. A., Solomko, Z. F., Pribega, L. V. Bromination of 4-(p-methoxyphenyl)-2,3-dihydro-1h-1, 5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1979, no. 6, р. 691-694.
Abronin, I. A., Gorb, L. G., Dryuk, V. G., Sheremet, V. I., Solomko, Z. F., Kremlev, M. M. Chlorination of 4-methyl-8-methoxy-2,3-dihydro-1H,1,5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1981, no. 9, р. 954-956.
Essassi, E. M., Viallefont, P., Zniber, R. Synthese et halogenation des cyclopropyl-4, 2H-dihydro-1,3-benzodiazepine-1,5 ones-2. Bull. Soc. Chim. France, 1986, no. 5. p. 797-800.
Solomko, Z. F., Proshkina, V. N., Bozhanova, N. Ya., Loban', S. V., Babichenko, L. N. Vilsmeier reaction with 4-phenyl-2,3-dihydro-1H-1,5-benzodiazepin-2-one. Chem. Heterocycl. Compd., 1984, no. 2, р. 183-185.
Solomko, Z. F., Proschkina, V. N., Avramenko, V. I., Plastun, I. A., Bozhanova, N. Ya. Formylation of 4-aryl-2,3-dihydro-1H-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1984, no. 9, р. 1035-1038.
Proschkina, V. N., Solomko, Z. F., Bozhanova, N. J. Features formylation of 8-methyl-4-phenyl-2,3-dihydro-1H-1,5-2-benzodiazepinone. Ukr. Khim. Zhurn., 1987, vol. 53, no. 9, p. 967-970.
Vernin, D., Domloi, H., Siv, C. Metzger, J., Archavlis, A., Llinas, J. R. Alkylation en catalyse par transfert de phase de dihydro-1,3(2H)benzo[2,3 b]diazepines-1,5-ones-2. Chem. Scripta, 1980, vol. 16, p. 157-162.
Gaponov, A. A. Reaction of 2,3-dihydro-1H-1,5-2-benzodiazepinones with alkylating agents. Visn. Dnipropetr. Univ.: Khim., 2010, no. 16, p. 99-104.
Bozhanov, V. I., Ivonin, S. I., Avramenko, V. I. Reaction of furoylacetic esther with aromatic diamines. Vopr. khimii i khim. tekhnologii, 2001, no. 1, p. 93-96.
Bozhanov, V. I., Ivonin, S. I. Synthesis of 4-Pyridyl-2,3-dihydro-1H-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 2002, no. 9, р. 1098-1103.
Achour, R., Essassi, E. M., Salem, M., Zniber, R. Etude de la reactivite du bis [phenyl-4-oxo-2-benzodiazepine-1,5-yl-1]-1,1’-methane. Bull. Soc. Chim. Belg., 1989. vol. 98, no. 6. p. 405-412.
Achour, R. Essassi, E. M., Zniber, R. Synthese et reactivite de nouvelles molecules de type bis-[phenyl-4 (cyclopropyl-4 et methyl-4)dihydro-2,3-oxo-2 benzodiazepine-1,5-yl-1]-1,n alcanes. Bull. Soc. Chim. France, 1988, no. 5, p. 889-896.
Essassi, E. M., Salem. M., Zniber, R.,. Viallefont, P., Gallo, R. Convenient phase transfer N-arylation of 1,3-dihydro-1,5-benzodiazepin-2-ones. Heterocycles, 1985, vol. 23, no. 4, p. 799-802.
Gaponov, A. A. Reaction of 4-phenyl-2,3-dihydro-1H-1,5-benzodiazepinone-2 with acetic anhydride. Visn. Dnipropetr. Univ.: Khim., 2004, no. 10, p. 62-64.
Gaponov, A. A. Acylation of 4-phenyl-2,3-dihydro-1H-1,5-benzodiazepinone-2. Visn. Dnipropetr. Univ.: Khim., 2001, no. 6, p. 64-66.
Sviatenko, L., Okovytyy, S., Gaponov, A., Kasyan, L., Tarabara, I., Leszczynski, J. DFT Study on Interaction of Acylation Reagents with 4-phenyl-1,3-dihydro-1,5-benzodiazepin-2-one. 17th Conference on Current Trends in Computational Chemistry : Abst. of Papers, Jackson, 2008, p. 161.
Puodzhyunaite, B. A., Yanchene, R. A., Terent'ev, P. B. Transformation of dihydro-1,5-benzodiazepin-2-ones under the influence of acetic anhydride. Chem. Heterocycl. Compd., 1988, no. 3, р. 311-317.
Motoki, S., Urakawa, C., Kano, A., Fushimi, Y., Hirano, T., Murata, K. Synthesis of benzodiazepine derivatives. Bull. Chem. Soc. Japan, 1970, vol. 43, p. 809 813.
Solomko, Z. F., Gaponov, A. A., Avramenko, V. I., Khmel', M. P. Synthesis of 4-styryl-2,3-dihydro-1H-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1987, no. 11, р. 1252-1254.
El-Shafei A. K., El-Kashef, H. S., El-Khawaga, A. M. Synthesis of 2-sybstituted 3-aryl / heteroaryl-2,3,3a,10-tetrahydro-4-methyl-pyrazolo[2,3-b][1,5] benzodiazepines. Indian J. Chem., 1982, vol. 21B, no. 7, p. 655-657.
Abdel-Ghany, H., El-Sayed, A. M., Sultan, A. A., El-Shafei, A. K. A novel syntheses of pyrano[2,3-c][1,5]benzodiazepines. Synth. Commun., 1990, vol. 20, no. 6, p. 893-900.
Solomko, Z. F., Chmel, M. P., Avramenko, V. I. Reaction of 2,3-dihydro-1H-1,5-benzodiazepinones-2 with o-nitrophenyl sulfenyl chloride. Ukr. Khim. Zhurn., 1991, vol. 57, no. 4, p. 423-426.
Gaponov, A. A., Solomko, Z. F., Bozhanova, N. Ya., Pantyukh, E. I. Recyclization of 4-methyl(phenyl)-2,3-dihydro-1h-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1989, no. 7, р. 836.
Gaponov, A. A., Solomko, Z. F., Tarabara, I. N. Reaction of 2,3-dihydro-1H-1,5-benzodiazepinonnes-2 with hydrazines. Vopr. khimii i khim. tekhnologii, 2007, no. 1, p. 24-27.
Gaponov, A. A., Okovytyy, S. I, Sviatenko, L. К., Kasyan L. I. Hydrazinolysis of 2,3-dihydro-1H-1,5-benzodiazepinonnes-2 and their thio analogues. Tezi dop. XXI Vseukr. konf. z org. khimii, Chernigiv, 2007, p. 128.
Svjatenko, L. K., Okovytyy, S. I., Gaponov, A. A., Tarabara, I. N., Leszczynski, J. Hydrazinolyses of 4-methyl-1,3-dihydro-2H-1,5-benzodiazepin-2-thione. 16th Conference on Current Trends in Computational Chemistry : Abstr. of Papers, Jackson, 2007, p. 206.
Okovytyy, S. I., Sviatenko, L. K., Gaponov, A. O., Tarabara, I. N., Kasyan, L. I., Leszczynski, J. Comprehensive DFT and MP2 level investigations of reaction of 2,3-dihydro-1,5-benzodiazepine-2-thiones with hydrazine. J. Phys. Chem. A, 2009, vol. 113, no. 42. p. 11376-11381.
Okovytyy, S. I., Sviatenko, L. K., Gaponov, A. O., Tarabara, I. N., Kasyan, L. I., Leszczynski, J. Theoretical Study of Mechanism of 2,3-Dihydro-1,5-benzodiazepin-2-ones Hydrazinolysis. J. Phys. Chem. A, 2009, vol. 113, no. 8, p. 1475 1480.
Essassi, E. M., Elabbassi, M., Fifani, J. Synthese des benzimidazolil-2 methil-5 pirazolil-3 methanes a partir des acetilmethylene-4-benzodiazepine-1,5-ones-2. Bull. Soc. Chim. Belg., 1987, vol. 96, no. 3. p. 225-228.
Essassi, E. M., M. Salem, M., Viallefont, P. Action de l`hydroxylamine sur les dihydro-1,3-benzodiazepine-1,5-thiones(ones)-2. Bull. Soc. Chim. France, 1987, no. 5, p. 890-892.
Dzvinchuk, I. B., Lozinskyy, M. O. The new direction of recycling the reaction of 4-methyl-1,3-dihydro-2H-1,5-benzodiazepin-2-one with benzoylhydrazine. Zh. Org. Khim., 1998, 34, no. 5, p. 782-783.
B. Capuano, B., Crosby, I. T., Lloyd, E. I., Taylor, D. Synthesis and preliminary pharmacological evaluation of 4-arylalkyl analoques of clozapine. Austral. J. Chem., 2007, vol. 60, no. 12, p. 928-933.
Aversa, M. C., Ferlazzo, A., Giannetto, P., Kohnke, F. H. A convenient synthesis of novel [1,2,4]triazolo[4,3-a][1,5]benzodiazepine. Synthesis, 1986, no. 3, p. 230-231.
Elhazazi, S., Baouid, A., Hasnaoui., A., Compain, P. Peri and regioselective synthesis of new heterocyclic compounds from 1,5-benzodiazepines. Synth. Commun., 2003, vol. 33, no. 1, p. 19-27.
Grossi, G. C., Roma, G., Di Braccio, M. Nuovi derivati [1,2,4]triazolo[4,3-a]-[1,5]benzodiazepinici farmacologicamente active. Chim. e ind., 1990, vol. 72, no. 11, p. 966-968.
Di Braccio, M., Roma, G., Grossi, G. C., Ghia, M., Mereto, E. Novel 4H-[1,2,4]triazolo[4,3-a] [1,5]benzodiazepine derivatives with analgesic or anti-inflammatory activity. Eur. J. Med. Chem., 1990, vol. 25, p. 681-687.
Roma, G., Grossi, G. C., Di Braccio, M., Ghia, M., Mattioli, F. A new route to substituted 4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepin-5-amines with analgesic and / or anti-inflammatory activities. Eur. J. Med. Chem., 1991, vol. 26, p. 489-496.
Gaponov, A. A. Reaction of 4-phenyl-1H-1,5-benzodiazepintione-2 with acetyl hydrazide. Visn. Dnipropetr. Univ.: Khim., 2012, no. 18, p. 101-105.
Kosychova, L., Stumbreviciute, Z., Pleckaitiene, L., Janciene, R., Puodziunaite, B. D. Synthesis of Substituted 5,6-Dihydro-4H-[1,2,4]triazolo[4,3-a]-[1,5]benzodiazepines. Chem. Heterocycl. Compd., 2004, no. 6, р. 811-815.
Chimirri, A., Grasso, S., Ottana, R., Romeo, G., Zappalà, M. Synthesis and stereochemistry of novel [1,2,4]oxadiazolo[4,5-a][1,5]-benzodiazepine derivatives. J. Heterocycl. Chem., 1990, vol. 27, no. 2, p. 371-374.
Cortes, E. C., Mellado, O. G., Cruz, E. H. Synthesis and properties of 5-methyl-4H-1-(p-substituted-phenyl)-3a-(p-substituted-phenyl)-9-[m-; and p-substituted)-phenoxy]-3a,4-dihydro[1,2,4]oxadiazolo[4,5-a][1,5]benzodiazepines. J. Heterocycl. Chem., 1999, vol. 36, p. 477-480.
El-Sayed, A. M., Abdel-Ghany, H., El-Saghier, A. M. A novel synthesis of pyrano(2,3-c)-, 1,3-oxazino(2,3-b)-, 1,2,4-triazolo(3,4-b)-, oxazolo(2,3-b)-, furano(3,2-c), and 3-substituted-(1,5)-benzodiazepine-2-ones. Synth. Commun., 1999, vol. 29, no. 20, p. 3561-3572.
Proshkina, V. N., Solomko, Z. F., Bozhanova, N. Ya. Formylation of 2,3-dihydro-4-methyl-1h-1,5-benzodiazepin-2-ones. Chem. Heterocycl. Compd., 1988, no. 9, р. 1071.
Puodziunaite, B., Janciene, R., Kosychova, L., Stumbreviciute, Z. On the synthetic way to novel peri-annelated imidazo [1,5] benzodiazepinones as the potent non-nucleoside reverse transcriptase inhibitors. Arkivoc, 2000, vol. 1, no. 4, p. 512-522.
Nardi, D., Tajana, A., Rossi, S. 4-Aryl-1,3-dihydro-2H-1,5-benzodiazepine-2-thiones. J. Heterocycl. Chem., 1973, vol. 10, p. 815-819.
Essassi, E. M., Salem, M. Synthese des pyrazolyl-1 benzimidazoles a partir des dihydro-1,3(2H)benzo[2,3-b]diazepine-1,5-ones-2. Bull. Soc. Chim. Belg., 1985, vol. 94, no. 10, p. 755-758.
Solomko, Z. F., Sharbatyan, P. A., Gaponov, A. A., Avraraenko, V. I. Synthesis and mass spectra of 2,3-dihydro-1H-1,5-benzodiazepine-2-thiones. Chem. Heterocycl. Compd., 1990, no. 3, р. 341-345.
Ayupova, A. T., adyrov, Ch. Sh., Seitanidi, K. Synthesis of substituted 1-isopropenyl- and 1-alkylbenzimidazolones. Chem. Heterocycl. Compd., 1974, no. 2, р. 207-209.
Kost, A. N., Solomko, Z. F., Budylin, V. A., Semenova, T. S. Synthesis of 5(and 6)-nitro-4-methyl-2,3-dihydro-1h-1,5-benzo-2-diazepinones. Chem. Heterocycl. Compd., 1972, no. 5, р. 632-635.
Pennini, R., Cerri, A., Tajana, A., Nardi, D., Giordano, F. Synthesis and structure determination of isomeric 7- and 8-chloro-1,5-benzodiazepine derivatives. J. Heterocycl. Chem., 1988, vol. 25, no. 1, p. 305-310.
Achour, R., Zniber, R. Syntheses des benzimidazolo [1,2-a] benzimidazoles a partir des benzodiazepine-1,5-ones-2. Bull. Soc. Chim. Belg., 1987, vol. 96, no. 10, p. 787-792.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

