Substituent and environment influence on analytical properties of associates of xanthene dyes with polyhexamethyleneguanidine chloride
DOI:
https://doi.org/10.15421/081320Keywords:
trioxyfluorone, spectrophotometry, polyhexamethyleneguanidine chloride, polyelectrolyte, ionisation constant, aggregationAbstract
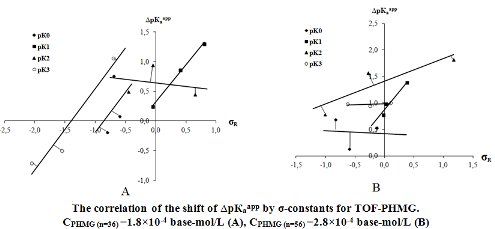
The interaction of 5 anionic xanthene dyes – 9-R-2,3,7-trihydroxy-6-fluorones (ТОF) with cationic polyelectrolyte (PE) – polyhexamethyleneguanidine chloride (PHMG) has been studied by the optical spectroscopy in a concentration range where PHMG has a polyelectrolyte effect. Hammet σ-constants were calculated. The correlations between structure of ТОF and shift of protolytic equilibrium in aqueous-polyelectrolyte solutions were established. Influence of рН, concentration of the ethanol, components ratio in the system PHMG-TOF on the character of the interaction of cationic PE with ТОF and value of the analytical signal were studied based on absorption spectra. The results were compared to those obtained in water solutions.
References
Brown, L., Halling, P. J., Johnston, G. A., Suckling, C. J., Valivety, R. H. The synthesis of some water insoluble dyes for the measurement of pH in water immiscible solvents. J. Chem. Soc. Perkin. Trans. 1, 1990, vol. 12, p. 3349-3353.
Kibblewhite, J., Drummond, C. J., Grieser, F., Thistlethwaite, P. J. Lipoidal eosin and fluorescein derivatives as probes of the electrostatic characteristics of self-assembled surfactant/water interfaces. J. Phys. Chem., 1989, vol. 93, no. 21, p. 7464 7473.
Neckers, D. C., Valdes-Aguilera, O. M. Photochemistry of the Xanthene Dyes. Adv. in Photochem., 1993, vol. 18, p. 315-394.
Choi, M. F., Hawkins, P. Solvatochromic Studies of Fluorescein Dianion in
N,N-Dimethylformamide/Water and Dimethylsulphoxide/Water Mixtures. Spectros. Lett., 1994, vol. 27, no. 8, p. 1049-1063.
Bilski, P., Holt, R. N., Chignell, C. F. Premicellar aggregates of Rose Bengal with cationic and zwitterionic surfactants. J. Photochem. and Photobiol. A: Chemistry, 1997, vol. 110, no. 1, p. 67-74.
Deshpande, A. V., Namdas, E. B. Spectroscopic properties of Na-fluorescein in polyacrylic acid films. J. Photochem. and Photobiol. A: Chemistry, 1997, vol. 110, no. 2, p. 177-182.
Blatt, E. Ground-state complexation of eosin-labeled fatty acids with dimethylaniline in cetyltrimethylammonium bromide micelles. J. Phys. Chem., 1986, vol. 90, no. 5, p. 874-877.
Chmilenko, T. S., Galimbievskа, E. A., Chmilenko, F. A. The formation ion associates by bromphenol red and their interaction with polyhexamethyleneguanidine in aqueous solutions. Methods and objects of chemical analysis, 2010, vol. 5, no. 1, p. 19-29.
Chmilenko, T. S., Tereshchenko, O. V., Chmilenko, F. A. Spectrophotometric study of aggregation of chlorphenol red in the presence of chloride polyhexamethyleneguanidine. Vopr. khimii i khim. tekhnologii, 2007, no. 5, p. 16-21.
Tanford, C. Physical chemistry of macromolecules, New York: Wiley, 1961. 772 p.
Chmilenko, F. A., Mikulenko, O. V., Chmilenko, T. S. The influence of polyvinylpyrrolidone on the chemical-analytical properties of phenylfluorone and its interaction with the uranium (VI). Vopr. khimii i khim. tekhnologii, 2003, no. 3, p. 44-47.
Nazarenko, V. A., Antonovich, V. P. Trioxyfluorones, Mockow: Nauka, 1973, 183 p.
Vodolazkaya, N. A., Mchedlov-Petrossyan, N. O., Bryleva, E. Yu., Biletskaya, S. V., Schrinner, M., Kutuzova, L. V., Ballauff, M. The binding ability and solvation properties of cationic spherical polyelectrolyte brushes as studied using acid-base and solvatochromic indicators. Functional Materials, 2010, vol. 17, no. 4, p. 470-476.
Savvin, S. B., Kuzin, E. L. Elektronnye spektry i struktura organicheskikh reagentov (Electronic Spectra and Structure of Organic Reagents), Moscow: Nauka, 1974, 277 p.
Byung-Soon, K., Daisuke, K., Young-A, S., Sung-Hoon, K., Shinya, M. Effect of phenyl ring substitution on J-aggregate formation ability of novel bisazomethine dyes in vapour-deposited films. Dyes and Pigments, 2011, vol. 90, no. 1, p. 56-64.
Dobrynin, A. V., Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci., 2005, vol. 30, p. 1049-1118.
Chmilenko, T. S., Kluchnik, L. A., Bohan, Yu. V., Chmilenko, F. A. Characteristic properties of salicylfluorone’s behavior in aqueous-polyelectrolyte solutions. Vopr. khimii i khim. tekhnologii, 2010, no. 6, p. 86-91.
Nyzhnyk V. V., Nyzhnyk T. Y., Malysheva M. A., Astrelin I. M. Association of metal ions with watersoluble polyhexamethyleneguanidine hydrochloride. Vopr. khimii i khim. tekhnologii, 2006, no. 6, p. 120-124.
Zhdanov, Y. А., Minkin V. I. The correlation analysis in organic chemistry, Rostov-na-Donu, 1966, 473 p.
Goldstein, D. J. Correlation of size of dye particle and density of substrate, with special reference to mucin staining. Stain Tech., 1962, vol. 37, p. 79-93.
Knof, J., Thiess, F.-J., Weber, J. Influence of aggregation on fluorescence decay of organic dyes. Naturforsch, 1978, vol. 33, p. 98-103.
Chambers, R. W., Kajiwara, Т., Keams, P. R. Effect of dimer formation on the Electronic Absorbtion and Emission Spectra of Xanthene Dyes. J. Phys. Chem., 1974, vol. 78, no. 4, p. 380-387.
Nooraldeen, A. Y., Palanichant, M., Palanisamy, P. K. Influence of Solvents Polarity on NLO Properties of Fluorone Dye. Int. J. Nonlinear Sci, 2009, vol. 7, no. 3, p. 290-300.
Burger, K. Solvation, Ionic and Complex Formation Reactions in Non-aqueous Solvents, Amsterdam: Elsevier, 1983, 192 p.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

