Properties of copper micropowders electroplated from sulfate solutions with acrylic acid or acrylamide
DOI:
https://doi.org/10.15421/081322Keywords:
micro particulates cathodic deposit, copper, acrylic acid, acrylamideAbstract
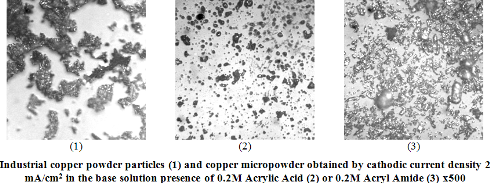
Based on the results of X-ray diffraction analysis it has been ascertained that cathodic copper deposit precipitated from sulfate electrolyte with acrylic acid or acrylamide consist of copper crystals with considerable quantity of imperfections and crystal deformations. It has been revealed that microstress and dislocation density in the deposits increase with enhancement of organic component concentration in the solution according to symbasis dependences. Decreasing of crystallite size and increasing of structuring degree for the deposits has been observed. Peaks of any phases except copper have not been identified on X-ray diffraction pattern of the deposits. In earlier investigation it has been shown that nonmetallic component (copper π-complexes) makes up 20% of the deposits. So based on the results of X-ray diffraction investigations we can conclude that the deposit consist of copper micro crystals separated by nonmetallic component. Owing to special physical-mechanical properties of the deposits it became possible to transform them to superfine powder by means of mechanical fragmentation. Using sedimentation and microscopic analysis it has been shown that cathodic micro powders precipitated from solutions with organic compounds are homogeneous by form and dimension. Significant bactericidal and bacteriostatic effects of cathodic micro powders influence have been detected.
References
Cotton, F., Wilkinson J. Sovremennaya neorganicheskaya khimiya, Ch. 3, Moscow: Mir, 1969, 542 p.
Vargaljuk, V. F., Polonskyy, V. A., Stets, N. V., Orlenko, O. S. Electrochemical formation and properties of copper precipitates obtained in the presence of acrylic acid. Visn. Dnipropetr. univ.: Khim, 2011, no. 17, p. 13-17.
Vargaljuk, V. F., Polonskyy, V. A., Stets, O. S., Shchukin, A. I. Effect of acrylic acid on the properties of copper coatings electrodeposited from sulfate solutions. Visn. Dnipropetr. univ.: Khim, 2012, no. 18, p. 15-18.
Vnukov, O. O., Chyhyrynets, O. E., Roslyk, I. H., Halchenko, H. Yu., Kabatska V. V. Ukraine Patent no. 201009579, 2010.
Karyakyn, Yu. V., Anhelov, I. I. Chistyye khimicheskiye veshchestva, Moscow: Khimia, 1974, 237 p.
Horelyk, S. S., Skakov, Yu. A., Rastorhuev, L. N. Rentgenograficheskiy i elektronno-opticheskiy analiz, Moscow: MISIS, 1994, 328 p.
Electrolytic copper powder. Spetifications. GOST 4960-75 (State standard), IPK Izdatelstvo standartov, 1975, 12 p.
Kratkiy spravochnik po khimii. Edited by Kurilenko, O. D., 1974, p. 821.
Spravochnik khimika. Ch. 1. Edited by Nykol'skyy B. P. et al. Moscow, Leningrad: Khimiya, 1966, p. 434.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

