Interaction of 2,3-dihydro-1H-benzodiazepinone-2 with epichlorhydrine
DOI:
https://doi.org/10.15421/081401Keywords:
1, 5-dihydrobenzodiazepinone-2, epichlorhydrine, structureAbstract
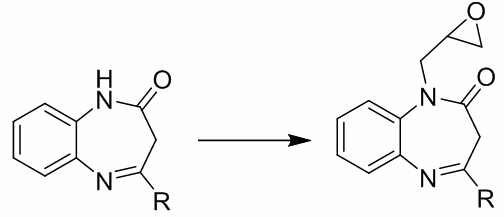
Derivatives of 1,5-benzodiazepines show various pharmacological activity. The presence of several reactive centers predetermines the possibility of reactions of 1,5-benzodiazepines both with electrophilic and nucleophilic reagents of various types. In addition, these compounds are suitable objects for the synthesis of new tricyclic systems containing a diazepinic cycle. In the article, the interaction of 4-phenyl- and 4-methyl-2,3-dihydro-1Н-1,5-benzodiazepine-2-ones with epichlorhydrin under various conditions is reported. It has been shown that under strongly basic conditions the reactions proceed at the nitrogen atom N1 and N1-glycidyl-1,5- benzodiazepine-2-ones are obtained. The yields of products were 71-79%. Under soft basic conditions the reactions either did not proceed at all or were accompanied by the formation of side-products.
References
Solomko, Z. F., Kost, A. N. 1,5-Benzodiazepines. Chem. Hetecycl. Compd., 1975, no. 11, р. 1231-1248.
Bogatskiy, A. V., Andronati, S. A., Golovenko, N. Ya. Trankvilizatoryi. 1,4- Benzodiazepinyi i rodstvennyie strukturyi, Kiev, Naukova dumka, 1980, 280 p. [in Russian]
Gaponov, A. A. Synthesis and biological activity of 2,3-dihydro-1H-1,5-benzodiazepinone-2. Visn. Dnipropetr. Univ.: Khim., 2012, vol. 20, no. 18, p. 82–90. [in Russian]
Chimirri, A., Grasso, S., Ottana, R., Romeo, G., Zappala, M. Synthesis and stereochemistry of novel [1,2,4]-oxadiazolo[4,5-a][1,5]-benzodiazepine derivatives. J. Heterocycl. Chem., 1990, vol. 27, no. 2, p. 371–374.
El-Sayed, A. M., Abdel-Ghany, H., El-Saghier, A. M. A novel synthesis of pyrano(2,3-c)-, 1,3-oxazino(2,3-b)-, 1,2,4-triazolo(3,4-b)-, oxazolo(2,3-b)-, furano(3,2-c)-, and 3-substituted-(1,5)-benzodiazepine-2-ones. Synth. Commun., 1999, vol. 29, no. 20, p. 3561–3572.
Rachmankulov, D. L., Kimsanov, B. X., Loktionov, N. A. Epichlorhydrin. Metodi sinteza, fizicheskiye i himicheskiye svoystva, tehnologiya proizvodstva. Moscow, Khimiya, 2003, 241 p. [in Russian]
Paquin, A. M. Epoxide compounds and epoxy resins. Berlin, Springer Verlag, 1958, 964 p.
Vernin, D., Domloi, H., Siv, C., Metzger, J., Archavlis, A., Llinas, J. R. Alkylation en catalyse par transfert de phase de dihydro-1,3-(2H)benzo[2,3b]diazepines-1,5-ones-2. Chem. Scripta, 1980, vol. 16, p. 157–162.
Gaponov, A. A. Reaction of 2,3-dihydro-1H-1,5-2-benzodiazepinonov with alkylating agents. Visn. Dnipropetr. Univ.: Khim., 2010, vol. 18, no. 16, p. 99–104. [in Russian]
Kost, A. N., Scharbatyan, P. A., Terent′yev, P. B., Solomko, Z. F., Tkachenko, V. S., Gergel, L. G. Mass-spektryi 1,5-benzodiazepinone-2. Zh. Org. Khim, 1972, vol. 8, no. 10, p. 2113–2119. [in Russian]
Lavergne, J.-P., Viallefont, P., Daunis, J. Recherches en serie azabenzodiazepine. Org. Mass. Spectrom., 1976, vol. 11, p. 680–696.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

