Statistical characteristics of spectrophotometric method for polyhexamethyleneguanidinedetermining
DOI:
https://doi.org/10.15421/081402Keywords:
spectrophotometry, trioxyfluorone, polyelectrolyte, metal-polymer complex, polyhexamethyleneguanidineAbstract
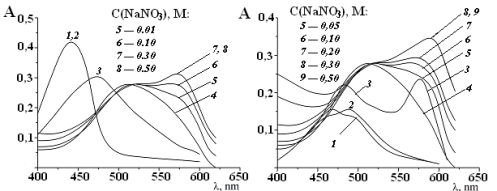
The interaction of two trioxyfluorone dyes disulfophenylfluorone and nitrophenylfluorone with Mo(VI) and polyhexamethyleneguanidine (PHMG) in the presense NO3–ions has been studied by optical spectroscopy. This ternary analytical system was proposed for determination of PHMG. Compositions of ternary complexes, formed in the system, were confirmed by the ternary Gibbs-Rosenbaum diagram. The optimal components ratio in complexes NPF:PHMG:Mo(VI) is 3:1:1 (at 520 nm) and 6:3:1(at 570 nm). The associate PHMG:NPF with ratio 1:2 and macrometalchelate PHMG·2Mo(VI) also records.The metals such asCu(II), V(IV), W(VI), Al(III), Co(II) do not interfere with the determination. Proposed technique allows to determine PHMG on second order nonlinear calibration curves in the concentration range of 0.1–2.0 mg/L. The limit of detection is 0.03 mg/L. Statistical treatment was carried out in accordance with ISO 8466:1-1990 and ISO 8466:2-1993.References
Water-Soluble Antimicrobial Preparation BIOR 1. State Technical Conditions TU 2499-001-36748375-97. [in Russian]
Instructions for use means "Dezavid" for disinfection of swimming pool water. – Moskow, 2005. – 20 p. [in Russian]
Rudnev, A. V., Dzherayan, T. G. Determination of polyhexamethyleneguanidine by capillary electrophoresis. J. Anal. Chem., 2006, vol. 61, no. 10, p. 1002–1005.
Dengbai, L., Jingui, L., Zhou, C., Luo, C. Polarographic Behavior of Co (II)–BSA or –HSA Complex in the Presence of a Guanidine Modifier. Anal. Chem., 2003, vol. 75, p. 6346–6350.
Kuman'ova, M. O., Golovej, O. P., Malec'kyj, M. M., Tkach, V. I., Determination of phosphate polyhexamethyleneguanidine by amperometric titration with 12-molybdophosphoric heteropolyacid. Vopr. Khim. Khim. Tekhnol., 2008, no. 1, p. 18–21. [in Ukrainian]
Chmilenko, F. A., Obrazcov, V. B., Korobova, I. V. Portretnyj, V. P., Danilenko, L. N. Amperometric determination of polyguanidine. Vopr. Khim. Khim. Tekhnol., 2001, no. 1, p. 40–42. [in Russian]
Matjushina, G. P., Popkov, V. A., Krasnjuk, I. K. Abrikosova, Ju. E. Quantitative Determination of Poly(Hexamethyleneguanidine) Salts Using the Fluorescence Quenching Technique. Pharm. Chem. J., 2005, no. 1, p. 50–52.
Chmilenko, T. S., Chmilenko, F. A. Analiticheskaja himija polijelektrolitov i ih primenenie v analize, Dnipropetrovsk: Izd-vo DNU, 2012, 224 p. [in Russian]
Chmylenko, T. S., Kljuchnyk, L. O., Pshedzjal, A. M., Chmylenko, F. O. Associate disulfophenylfluorone with polyhexamethyleneguanidine as analytical form for determination of vitamin В12. Vopr. Khim. Khim. Tekhnol., 2011, no. 5, p. 85–89. [in Ukrainian]
Chmilenko, T. S., Ivanitsa, L. A., Chmilenko, F. A. Spectrophotometric determination of zineb using disulfophenylfluorone and polyhexamethyleneguanidine chloride. Visn. Khark. nats. univ., 2012, № 1026. Ser. Khim., no. 21 (44), P. 243–250. [in Russian]
Lileev, A. S., Lyashchenko, A. K., Ostroushko, A. A., Sennikov, M. Yu. Dielectric properties of aqueous solutions of the ammonium heptamolybdate-poly(vinyl alcohol)-water system. J. Inorg. Chem., 2006, vol. 51, no. 4, p. 656–661.
Nazarenko, V. A., Antonovich, V. P. Trioksifluorony. Moscow: Nauka, 1973, 182 p. [in Russian]
Gembickij, P. A., Voinceva, I. I. Polimernyj biocidnyj preparat poligeksametilenguanidin. Zaporozh'e: Poligraf, 1998, 44 p. [in Russian]
Korostelev, P. P. Reaktivy dlja tehnicheskogo analiza. Moscow: Metallurgija, 1988, 382 p. [in Russian]
Busev, A. I. Analiticheskaja himija molibdena. Moscow: Izd-vo AN SSSR, 1962, 302 p. [in Russian]
Funk, W., Dammann, V., Donnevert, G. Quality Assurance in Analytical Chemistry. Second, Completely Revised and Enlarged Edition, Weinheim: Wiley-VCH, 2007, 277 p.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

