Microextraction separation, preconcentration and spectrophotometric determination of sodium dodecyl sulfate as an ion associate with quinaldine red
DOI:
https://doi.org/10.15421/081404Keywords:
microextraction, sodium dodecyl sulfate, spectrophotometric determination, Quinaldine RedAbstract
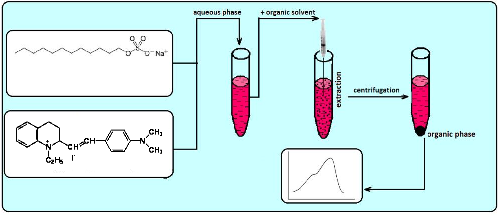
The optimal conditions for the microextraction separation, preconcentration and spectrophotometric determination of sodium dodecyl sulfate (SDS) as an its ion associate (IA) with Quinaldine Red (QR) have been studied. Was tested a large number of organic solvents as extractants. Aliphatic hydrocarbons (hexane) extracted IA considerably weaker than, halogen and nitro derivatives of hydrocarbons (chlorobenzene, bromobenzene, nitrobenzene, chloroform, dichloroethane), extracted with IA and the simple salt of the dye. The best solvent found for the extraction of SDS was mixture of carbon tetrachloride with dichloroethane or chloroform that provided 10 to 50 fold concentration of SDS by microvolume of organic phase. The maximum extraction of SDS was achieved in the concentration range of QR 1.0∙10–4 mol/l of QR after which the optical density does not change practically (excess dye remains in the aqueous phase). The dye of QR is highly stable in an alkaline environment, it can be used for the extraction of SDS in a wide pH range and rely on high selectivity determination. The pH range for maximum extraction of ion associate was 4–12. We found that 50000–100000-fold amounts F–, Cl–, Br–, NO2–, HCO3–, CH3COO–, SO42–, 10000–20000-fold amounts NO3–, I–, HPO42–, B4O72–, IO3–, ClO3–, C2O42–, 300-fold amounts ClO4– do not interfere with the determination of SDS. The molar ratio of SDS and QR determined by various spectrophotometric methods (isomolar series, Asmus, equilibrium shift) is 1:1. The limit of detection was 0.04 µg/ml. A new method of extraction-spectrophotometric determination was applied to the determination of anionic surfactants in various wastewater samples.
References
Water. Rates of measurement error of characteristics of composition and properties. GOST (Interstate standard) 27384-2002, IPK Izdatelstvo standartov, 2002, 10 p. [in Russian]
Bazel, Ya. R., Antal, I. P., Lavra, V. M., Kormosh, Zh. A. Methods for the determination of anionic surfactants. J. Anal. Chem., 2014, vol. 69, no 3, p. 211–236.
Kamaya, M., Tomizawa, Y., Nagashima, K. Spectrophotometric method for the determination of an anionic surfactant without liquid-liquid extraction. Anal. Chim. Acta., 1998, vol. 362, no 2–3, p. 157–161.
Gao, H.-W., Zhao, J.-F. Langmuir aggregation of thionin on sodium dodecyl sulfate and its application. J. Anal. Chem., 2003, vol. 58, no 4, p. 322–327.
Dolenko, S. A., Alekseenko, E. Yu., Kuschevskaya, N. F. Sorption–photometric determination of anionic surfactants in water. J. Anal. Chem., 2010, vol. 65, no 3, p. 229–233.
Motomizu, S., Kobayashi, M. Flow-injection method for the determination of anionic surfactants after liquid-liquid extraction using on-tube visible absorption and fluorescence detection. Anal. Chim. Acta., 1992, vol. 261, no 1–2, p. 471–475.
Sakai, T., Harada, H., Liu, Х., Ura, N., Takeyoshi, K., Sugimoto, Ku. New phase separator for extraction-spectrophotometric determination of anionic surfactants with malachite green by flow-injection analysis. Talanta, 1998, vol. 45, no 3, p. 543–548.
Patel, R., Patel, K.S. Flow injection determination of anionic surfactants with cationic dyes in water bodies of central India. Analyst, 1998, vol. 123, no 8, p. 1691–1695.
Drinking water. Methods for determination of surfactants. GOST (State standard) R 51211-98, IPK Izdatelstvo standartov, 1998, 15 p. [in Russian]
ISO 7875-1:1996 Water quality – Determination of surfactants – Part 1: Determination of anionic surfactants by measurement of the methylene blue index (MBAS). ISO/TC 147. International Organization for Standardization, Geneva, Switzerland.
Liu, J.-F., Jiang, G.-B. Determination of anionic surfactants in detergents by microporous membrane liquid-liquid extraction and flow injection spectrophotometry. Microchem. J., 2001, vol. 68, no 1, p. 29–33.
Liu, J. Flow injection determination of anionic surfactants based on the solvatochromism of p-diphenylaminoazobenzene sulfonate. Anal. Chim. Acta, 1997, vol. 343, no 1–2, p. 33–37.
Patel, R., Patel, K. S. Simple and specific method for flow injection analysis determination of cationic surfactants in environmental and commodity samples. Talanta, 1999, vol. 48, p. 923–931.
Moskvin, L. N., Simon, J., Löffler, P., Michailova, N. V., Nicolaeva, D. N. Photometric determination of anionic surfactants with flow-injection analyzer that includes a chromatomembrane cell for sample preconcentration by liquid-liquid solvent extraction. Talanta, 1996, vol. 43, no 6, p. 819–824.
Škrlíková, J., Andruch, V., Balogh, I. S., Kocúrová, L., Nagy, L., Bazeľ, Y. A novel, environmentally friendly dispersive liquid-liquid microextraction procedure for determination of copper. Microchem. J., 2011, vol. 99, no. 1, p. 40–45.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

