Simultaneous determination of two active components of pharmaceutical preparations by sequential injection method using heteropoly complexes
DOI:
https://doi.org/10.15421/081408Keywords:
spectrophotometric determination, rutin, ascorbic acid, 18-molybdo-2-phosphate heteropoly anionAbstract
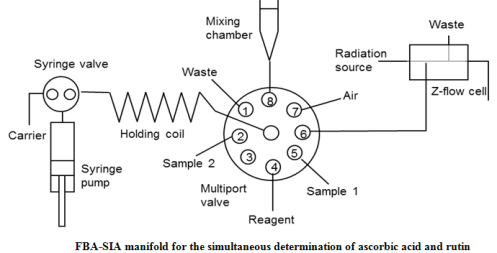
New approach has been proposed for the simultaneous determination of two reducing agents based on the dependence of their reaction rate with 18-molybdo-2-phosphate heteropoly complex on pH. The method was automated using the manifold typical for the sequential analysis method. Ascorbic acid and rutin were determined by successive injection of two samples acidified to different pH. The linear range for rutin determination was 0.6-20 mg/L and the detection limit was 0.2 mg/L (l = 1 cm). The determination of rutin was possible in the presence of up to a 20-fold excess of ascorbic acid. The method was successfully applied to the determination of ascorbic acid and rutin in ascorutin tablets. The applicability of the proposed method for the determination of total polyphenol content in natural plant samples was shown.
References
Burguera, J. L., Burguera, M. Flow injection-electrothermal atomic absorption spectrometry configurations: recent developments and trends. Spectrochim. Acta B, 2001, vol. 56, no. 10, p. 1801–1829.
Gomez, V., Callao, M. P. Multicomponent analysis using flow systems. Trends Anal. Chem., 2007, vol. 26, p. 767–774.
Luque de Castro, M. D., Valcarcel, M. Flow injection methods based on multidetection. Trends Anal. Chem., 1986, vol. 5, p. 71–74.
Bulatov, A. V., Petrova, A. V., Vishnikin, A. B., Moskvin, A. L., Moskvin, L. N. Stepwise injection spectrophotometric determination of epinephrine. Talanta, 2012, vol. 96, p. 62–67.
Diniz, P. H. G. D., Almeida, L. F., Harding, D. P., Araújo, M. C. U. Flow-batch analysis. Trends Anal. Chem., 2012, vol. 35, p. 39–49.
Vishnikin, A. B., Al-Shwaiyat, M. K. E. A., Petrushina, G. A., Tsiganok, L. P., Andruch, V., Bazel, Ya. R., Sklenařova, H., Solich, P. Highly sensitive sequential injection determination of p-aminophenol in paracetamol formulations with 18-molybdodiphosphate heteropoly anion based on elimination of Schlieren effect. Talanta, 2012, vol. 96, p. 230–235.
Zaporozhets, O. A., Krushinskaya, Е. А. Determination of ascorbic acid by molecular spectroscopic techniques. J. Anal. Chem., 2002, vol. 57, no. 4, p. 286–297.
Vishnikin, A. B., Sklenařova, H., Solich, P., Petrushina, G. A., Tsiganok, L. P. Determination of ascorbic acid with Wells-Dawson type molybdophosphate in sequential injection system. Anal. Lett., 2011, vol. 44, no. 1–3, p. 514–527.
Bulatov, A. V., Petrova, A. V., Vishnikin, A. B., Moskvin, L. N. Stepwise injection spectrophotometric determination of cysteine in biologically active supplements and fodders. Microchem. J., 2013, vol. 108, p. 213–217.
Petrushina, G. A., Tsiganok, L. P., Vishnikin, A. B. Spectrophotometric determination of p-aminophenol in the presence of paracetamol by using 18-molybdodiphosphate. Visn. Dnipropetr. Univ.: Khim., 2011, vol. 19, no. 17, p. 160–164. [in Russian]
Al-Shwaiyat, M. K. E. A., Vishnikin, A. B., Tsiganok, L. P., Kabashnaya, E. V., Khmelovskaya, S. A., Andruch, V., Bazel, Ya. R., Sklenářová, H., Solich, P. Sequential injection spectrophotometric determination of analgine in pharmaceutical formulations using 18-molybdo-2-phosphate heteropoly anion as chromogenic reagent. Visn. Dnipropetr. Univ.: Khim., 2013, vol. 21, no, 19, p. 7–18.
Petrushina, G. A., Tsiganok, L. P., Vishnikin, A. B. Spectrophotometric determination of ascorbic acid by using P2Mo18O626–. Ukr. Chem. J., 2010, vol. 76, no. 4, p. 116–122.
Honorato, R. S., Araújo, M. C. U., Lima, R. A. C., Zagatto, E. A. G., Lapa, R. A. S., Costa Lima, J. L. F. A flow-batch titrator exploiting a one-dimensional optimisation algorithm for end point search. Anal. Chim. Acta, 1999, vol. 396, p. 91–97.
Zagatto, E. A. G., Carneiro, J. M. T., Vicente, S., Fortes, P. R., Santos, J. L. M., Lima, J. L. F. C. Mixing chambers in flow analysis: a review. J. Anal. Chem., 2009, vol. 64, no. 5, p. 524–532.
Mozzhukhin, A. V., Moskvin, A. L., Moskvin, L. N. Stepwise injection analysis as a new method of flow analysis. J. Anal. Chem., 2007, vol. 62, no. 5, p. 475–478.
Bulatov, A. V., Moskvin, A. L., Moskvin, L. N., Mozhuhin, A. V. The stepwise injection analysis as a new opportunity for automation of chemical analysis of liquid, gaseous and solid-phase samples. J. Flow Inject. Anal., 2010, vol. 27, no. 1, p. 13–19.
Ruzicka, J. Flow injection analysis. 2nd Ed., New York: Wiley-Interscience, 1988, 507 p.
Li, X., Zhang, Y. Separation and determination of rutin and quercetin in the flowers of Sophora japonica by capillary electrophoresis with electrochemical detection. Chromatographia, 2002, vol. 55, no. 3/4, p. 243–246.
Ding, S., Dudley, E., Plummer, S., Tang, J., Newton, R. P., Brenton, A. G. Quantitative determination of major active components in Gingko biloba dietary supplements by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom., 2006, vol. 20, p. 2753–2760.
AOAC. Official methods of analysis of the association of official analytical chemists. – Washington D.C., USA: AOAC, 1990., P. 135.
Zaporozhets, O. A., Krushinska, O. A., Lipkovska, N. A., Barvichenko, V. N. A new test method for the evaluation of total antioxidant activity of herbal products. J. Agric. Food. Chem., 2004, vol. 52, p. 21–25.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

