Microbiological properties of copper dispersion obtained by cathodic deposition in the presence of acrylic acid
DOI:
https://doi.org/10.15421/081420Keywords:
micropowder, copper, acrylic acid, microbiological activityAbstract
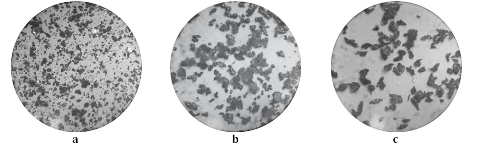
Quantitative composition of organometallic electrodeposit obtained from the solution of 0.1 M CuSO4, 1.0 M H2SO4 and 0.2 M acrylic acid was determined by differential photometry. It was shown that the deposit contains copper complexes in an amount of 15% (wt.). Using the dispersion grain-size and microscopic analysis it was ascertained that the micropowder obtained by mechanical grinding of organometallic electrodeposit is more fine-grained and homogeneous compared with industrial or chemically obtained (by cementation with zinc) copper powders. Microbiological studies using clinical strain of microorganisms Staphylococcus aureus showed that the obtained copper-acrylate micropowder has bacteriostatic and bactericidal action. Suppression of bacteria’s vital activity in the interaction with the organometallic dispersion occurs from the first minute of exposure in contrast to the influence of industrial and chemically produced copper powders. This effect is related to the special structure of copper-acrylate powder.
References
Elechiguerra, J. L., Burt, J. L., Morones, J. R., Camacho-Bragado A., Gao X., Lara H. H., Yacaman M. J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology, 2005, 3:6.
Vargalyuk, V. F., Polonskyy, V. A., Orlenko, O. S. Quantum chemical study on the impact of olefin compounds electro-reduction process copper ions Nauk. Visn. Chern. Univ.: Khim, 2008, no. 399–400, p. 183–185. [in Ukrainian]
Fisher, E., Werner, G. Metal π-Complexes. New York: Elsevier publ. 1968, 264 p.
Cotton, A. Wilkinson, G. Advanced Inorganic Chemistry, 1969, V. 3, 593 p.
Vargalyuk, V. F., Polonskyy, V. A., Stets, N. V., Orlenko, O. S. Electrochemical formation and properties of copper precipitates obtained in the presence of acrylic acid. Visn. Dnipropetr. Univ.: Khim., 2011, vol. 19, no. 17, p. 13–17. [in Ukrainian]
Vargalyuk, V. F., Polonskyy, V. A., Stets, O. S. Properties micron copper electrodeposited from sulfate solutions containing acrylic acid or acrylamide Visn. Dnipropetr. Univ.: Khim., 2013, vol. 21, no. 20, p. 90–96. [in Ukrainian]
Electrolytic copper powder. Specifications. GOST (USSR standard) 4960-75, IPK Izdatelstvo standartov, 1975, 12 p. [in Russian]
Vnukov, O. O., Chyhyrynetsʹ, O. E., Roslyk, I. H., Halchenko, H. Yu., Kabatska V. V. Ukraine Patent no. 56876, 2010. [in Ukrainian]
Karyakyn, Yu. V., Anhelov I. I. Pure chemical substances. 1974, p. 237. [in Russian]
Hodakov, G. S., Yudkin, Y.P. Sedimentation analysis of superfine systems. 1981, p. 51. [in Russian]
Malkina, T. G., Podchajnova, V. H. Definition of large amounts of copper by differential photometry. Zh. Anal. Khim., 1964, vol. 19, no. 6, p. 668–670. [in Russian]
Vargalyuk, V. F., Polonskyy, V. A., Stets, O. S., Balalayev, O. K. Structure and properties of copper coatings electrodeposited from sulfuric acid solutions containing acrylic acid and acrylic amide. Ukr. Chem. J., 2013, vol. 79, no. 3, p. 51–58. [in Ukrainian]
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

