Theoretical study on alkaline hydrolysis of trinitrotoluene: later steps
DOI:
https://doi.org/10.15421/081501Keywords:
trinitrotoluene, DFT, hydrolysis, mechanismAbstract
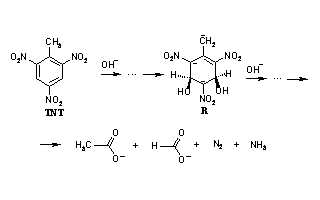
Alkaline hydrolysis is an effective method to destroy such the pollutant as 2,4,6-trinitrotoluene (TNT) in solution and in well-mixed soil. The mechanism of hydrolytic transformation of polynegative complex, which is one of the products of early stages of TNT hydrolysis, was theoretically investigated at the SMD(Pauling)/M06-2X/6-31+G(d,p) level under alkali condition. The studied process consists of more than twenty steps and includes a six-membered cycle cleavage and sequenced [1,3]-hydrogen migration and C-C bond rupture. The highest energy barrier is observed for interaction of nitromethanide with hydroxide. The most exothermic steps are C–C bonds breaking. As a result final products such as formate, acetate, ammonium, and nitrogen are formed.References
U.S. Department of Health and Human Services (1995). Toxicological profile for 2,4,6-trinitrotoluene (TNT). Retrieved from http://www.atsdr.cdc.gov/-toxprofiles/tp81.pdf
Dodd, D. E., & McDougal, J. N. (2002). Recommendation of an Occupational Exposure Level for PAX-21. AFRL-HE-WP-TR-2001-0103. Wright-Patterson Air Force Base: Air Force Research Laboratory.
Jarvis, A. S., McFarland, V. A., & Honeycutt, M. E. (1998). Assessment of the effectiveness of composting for the reduction of toxicity and mutagenicity of explosive-contaminated soil. Ecotoxicol. Environ. Saf., 39, 131–132.
Tchounwou, P. B., Newsome, C., Glass, K., Centeno, J. A., Leszczynski, J., Bryant, J., Okoh, J., Ishaque, A., & Brower, M. (2003). Environmental toxicology and health effects associated with dinitrotoluene exposure. Rev. Environ. Health, 18, 203–229.
Felt, D. R., Nestler, C. C., Davis, J. L., & Larson, S. L. (2007). Potential for Biodegradation of the Alkaline Hydrolysis End Products of TNT and RDX. ERDC/EL TR-07-25. Vicksburg: U.S. Army Engineer Research and Development Center, Environmental Laboratory.
Emmrich, M. (1999). Kinetics of the Alkaline Hydrolysis of 2,4,6-Trinitrotoluene in Aqueous Solution and Highly Contaminated Soils. Environ. Sci. Technol., 33, 3802–3805.
Emmrich, M. (2001). Kinetics of the Alkaline Hydrolysis of Important Nitroaromatic Co-contaminants of 2,4,6-Trinitrotoluene in Highly Contaminated Soils. Environ. Sci. Technol., 35, 874–877.
Mills, A., Seth, A., & Peters, G. (2003). Alkaline hydrolysis of trinitrotoluene, TNT. Phys. Chem. Chem. Phys., 5, 3921–3927.
Felt, D. R., Larson, S. L., & Hansen, L. D. (2001). The molecular weight distribution of the final products of TNT-hydroxide reaction. ERDC/EL TR-01-16. Vicksburg: U.S. Army Engineer Research and Development Center, Environmental Laboratory.
Felt, D. R., Larson, S. L., & Valente, E. J. (2002). UV-Vis spectroscopy of 2,4,6-trinitrotoluene-hydroxide reaction. ERDC/EL TR-02-22. Vicksburg: U.S. Army Engineer Research and Development Center, Environmental Laboratory.
Bishop, R. L., Flesner, R. L., Larson, S. A., & Bell, D. A. (2000). Base hydrolysis of TNT-Based explosives. J. Energ. Mater., 18(4), 275–288.
Saupe, A., Garvens, H. J., & Heinze, L. (1998). Alkaline hydrolysis of TNT and TNT in soil followed by thermal treatment of the hydrolysates. Chemosphere, 36, 1725–1744.
Thorn, K. A., Thorne, P. G., & Cox, L. G. (2004). Alkaline Hydrolysis/Polymerization of 2,4,6-Trinitrotoluene: Characterization of Products by 13C and 15N NMR. Environ. Sci. Technol., 38, 2224–2231.
Davis, J. L., Nestler, C. C., Felt, D. R., & Larson, S. L. (2007). Effect of Treatment pH on the End Products of the Alkaline Hydrolysis of TNT and RDX. ERDC/EL TR-07-4. Vicksburg: U.S. Army Engineer Research and Development Center, Environmental Laboratory.
Hill, F. C., Sviatenko, L. K., Gorb, L., Okovytyy, S. I., Blaustein, G. S., & Leszczynski, J. (2012). DFT M06-2X investigation of alkaline hydrolysis of nitroaromatic compounds. Chemosphere, 88, 635–643.
Sviatenko, L. K., Kinney, C. A., Gorb, L., Hill, F. C., Bednar, A. J., Okovyty, S. I., & Leszczynski, J. (2014). Comprehensive investigations of kinetics of alkaline hydrolysis of TNT (2,4,6-trinitrotoluene), DNT (2,4-dinitrotoluene) and DNAN (2,4-dinitroanisole). Environ. Sci. Technol., 48(17), 10465–10474.
Salter-Blanc, A. J., Bylaska, E. J., Ritchie, J. J., & Tratnyek, P. G. (2013). Mechanisms and Kinetics of Alkaline Hydrolysis of the Energetic Nitroaromatic Compounds 2,4,6-Trinitrotoluene (TNT) and 2,4-Dinitroanisole (DNAN). Environ. Sci. Technol., 47, 6790–6798.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr., J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2009). Gaussian 09 (Revision A.02) [Computer software]. Gaussian Inc., Wallingford CT.
Hehre, W. J., Radom, L., Schleyer, Pv. R., & Pople, J. A. (1986). Ab Initio Molecular Orbital Theory. New York, USA: Wiley.
Zhao, Y., & Truhlar, D.G. (2006). A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys., 125, 194101–194118.
Wei C. (2005). Thermal Runaway Reaction Hazard and Decomposition Mechanism of hydroxylamine system. (Doctoral dissertation). Retrieved from http://pscfiles.tamu.edu/library/center-publications/theses-and-dissertations/chunyangwei.pdf.
Downloads
Published
Issue
Section
License
Copyright (c) 2015 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

