The quantum-chemical investigation of structure and spectral characteristics for molecular complexes in systems «polyarylate-terlon» and «penton-terlon»: a comparative analysis
DOI:
https://doi.org/10.15421/081503Keywords:
ab initio calculations, intermolecular interaction, hydrogen bond, stabilization energy, basis set superposition error, vibrational spectraAbstract
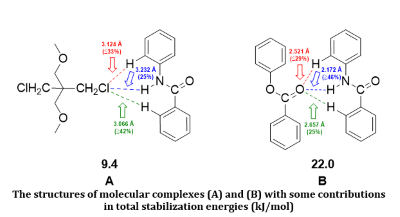
The structural and energetic parameters for molecular complexes of phenylbenzoate with N-phenylbenzamide are reflected in some features of intermolecular interaction in system «polyarylate-terlon» at DFT-B3LYP/6-311++G(d,p) level of theory. In this case the total stabilization energy effects have been achieved mainly by formation of some typical hydrogen bonds, which are realized between oxygen atom of carbonyl group and hydrogen atoms of benzene rings with some closed of amide fragment. By comparative analysis of calculation results with that data, which have been obtained for relative system «penton-terlon», a great advantage of electrostatic interaction forces in total stabilization effects have also been proved for molecular complexes. The proposed theoretical models are validated in reflection of spectral and energetic characteristics for investigating systems.References
Burya, A. I., Chigvintseva, O. P., & Suchilina-Sokolenko, S. P. (2001). [Polyarylates. Synthesis, properties, composition materials]. Dnepropetrovsk, Ukraine: Nauka i osvita (in Russian).
Dolbin, I. V., Burya, A. I., & Kozlov, G. V. (2005). [Correlation of structure and thermal properties of composites based on polyarylate]. Fundamentalnyie issledovaniya, (3), 39–41 (in Russian).
Redchuk, A. S., Burya, A. I., & Golovyatinskaya, V. V. (2011). [IR spectra and X-ray analysis of composites based on pentaplast filled with terlon fiber]. Kompozitnyie materialyi, 5(2), 59–65 (in Russian).
Holtje, H.-D., Sippl, W., Rognan, D., & Folkers, G. (2008). Molecular Modeling. Basic Principles and Applications. Weinheim, Federal Republic of Germany: WILEY-VCH Verlag GmbH & Co. KGaA.
Tokar, A. V., & Chigvintseva, O. P. (2013). The quantum-chemical modeling of structure and spectral characteristics for molecular complexes in system «penton-terlon». Visn. Dnipropetr. Univ.: Khim. – Bull. Dnipropetr. Univ.: Chem., 21(20), 44–49 (in Russian).
Kolandaivel, P., & Nirmala, V. (2004). Study of proper and improper hydrogen bonding using Bader’s atoms in molecules (AIM) theory and NBO analysis. J. Mol. Struct., 694(1–3), 33–38.
Weinhold, F. (2012). Natural bond orbital analysis: A critical overview of relationships to alternative bonding perspectives. J. Comput. Chem., 33(30), 2363–2379.
Simon, S., Duran, M., & Dannenberg, J. J. (1996). How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys., 105(24), 11024–11031.
Sordo, J. A. (2001). On the use of the Boys-Bernardi function counterpoise procedure to correct barrier heights for basis set superposition error. J. Mol. Struct., 537(1–3), 245-251.
Tognetti, V. & Joubert, L. (2014). Density functional theory and Bader’s atoms-in-molecules theory: towards a vivid dialogue. Phys. Chem. Chem. Phys., 16(28), 14539–14550.
Tokar, A. V., & Chigvintseva, O. P. (2014). The quantum-chemical modeling of structure and spectral characteristics for molecular complexes in systems «penton-terlon» and «polyarylate-terlon»: a comparative analysis. 10th Saint-Petersburg Young Scientists Conference «Modern problems of polymer science» (p. 43). Saint-Petersburg, Russian Federation.
Butyirskaya, E. V. (2011). [Computational chemistry: bases of theory and work with the programs of Gaussian and GaussView]. Moscow, Russian Federation: SOLON-PRESS (in Russian).
systems «penton-terlon» and «polyarylate-terlon»: a comparative analysis / A. V. Tokar, O. P. Chigvintseva // 10th Saint-Petersburg Young Scientists Conference.
Downloads
Published
Issue
Section
License
Copyright (c) 2015 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

