The HN- and N-methylimines of formaldehyde, acetaldehyde and acetone: electronic structure and inversion barriers of nitrogen atom
DOI:
https://doi.org/10.15421/081504Keywords:
imines, inversion, electron density, intramolecular interactionsAbstract
The transmission of electronic effects and factors which affect the inversion barriers of atom N (ΔEi¹) in unsubstituted (I) and N-methyl derivatives (II) of formaldehyde (a), acetaldehyde (b), and acetone (c) have been investigated using the DFT method within the NBO. It was defined that the negative charge on the N atom (qN) decreases with N-methylation. The energy of lone pare, qN and ΔEi¹ values (imines Ia, E-Ib; Z-Ib, Ic; IIa E-IIb; Z-IIb, IIc) increases and the negative charge on C= atom decreases with C-methylation. The reduction of qN values in N-methylimines is caused by the major influence of more electronegativity of C atom and minor influence of two-electronic interactions. The relative increase of qN values with increase of degree of C-methylation is caused by hyperconjugation of the methyl groups with the antibonding orbitals of C=N bond but not +I-effects of C-substituents. C-methylation increases the inversion barriers owing to the interactions of the orbitals of iminyl atom C and does not affect their changes due to the interactions involving orbitals of =C–R2 bonds. It denies the supposed dominant influence of nN®s*C–R interactions on ΔEi¹ values. In general, electronegativity of N-substituents and steric strain has the dominant influence on the inversion barriers but not the intramolecular interactions.References
Lehn, J. M. (1970). Nitrogen inversion experiment and theory. Top. Curr. Chem., 15(3), 311–377.
Kessler, H. (1970). Detection of Hindered Rotation and Inversion by NMR Spectroscopy. Angew. Chem., Int. Ed. Engl., 9(3), 219–235.
Raban, M. (1970). Investigation of the mechanism of syn–anti-isomerism in imines using CNDO/2 calculations. Chem. Commun. 1415–1416.
Wurmb-Gerlich, D., Vögtle F., Mannschreck, A., & Staab, H. A. (1967). The study of syn-anti Isomerization of Imines by proton magnetic resonance. Justus Liebigs Ann. Chem., 708, 36–50.
Jennings, W. B., & Boyd, D. R. (1972). The Mechanism of Interconversion of (Z)- and (E)-Ketimines. J. Am. Chem. Soc., 94(20), 7187–7188.
Jennings, W. B., Al-Showiman, S., Boyd, D. R., & Campbell, R. M. (1976). Dynamic Stereochemistry of lmines and Derivatives. Part IX. The Mechanism of E-Z Isomerization in N-Alkylimines. J. Chem. Soc., Perkin Trans. 2, (13), 1501–1506.
Lehn, J. M., & Munsch, B. (1968). An ab initio SCF-LCAO-MO Study of the Nitrogen Inversion Barriers in Methylenimine, Diimide and Carbodiimide. Theor. Chim. Acta, 12, 91–94.
Brown, C., Grayson, B. T., & Hudson, R. F. (1979). Z-E isomerisation of N-sulphenylimines. J. Chem. Soc., Perkin Trans. 2, (4), 427–434.
Dawson, W. H., Hunter, D. H., & Willis, C. J. (1980). Syn-anti- Isomerization of the N-Heptafluoroisopropylimine of Hexafluoroacetone; Steric Effects or Negative Hyperconjugation? J. Chem. Soc., Chem. Commun., (18), 874–875.
Prosyanik, A. V. (1987). [Synthesis and stereochemistry of imines and aziridines]. (Unpublished doctoral dissertation). Ukrainian State University of Chemical Technology, Dnipropetrovsk, Ukraine (in Russian).
Prosyanik, A. V., Afanasiev, D. Yu., & Fedoseyenko, D. V. (2004). Theoretical Studies on Z, E-Isomerization of HN- and N-Alkylimines (p. 141-144). Current Trends in Computational Chemistry. Jackson, USA.
He, S., Tan, Y., Xiao, X., Zhu, L., Guo, Y., Li, M., Tian, A., Pu, X., & Wong, N. B. (2010). Substituent effects on electronic character of the C=N group and trans/cis isomerization in the C-substituted imine derivatives: A computational study. J. Mol. Struct.: THEOCHEM, 951, 7–13.
Kutsik-Savchenko, N. V., Lebed, O. S., & Prosyanik, A. V. (2014). NH- and N-alkylformaldimines: the electronic structure and inversion barriers of nitrogen atom. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (1), 15–23 (in Russian).
Weigend, F., & Ahlrichs, R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys., 7, 3297–3305.
Schmidt, M. W., Baldridge, K. K., Boatz, J. A., Elbert, S. T., Gordon, M. S., Jensen, J. H., Koseki, S., Matsunaga, N., Nguyen, K. A., Su, S. J., Windus, T. L., Dupuis, M., & Montgomery, J. A. (1993). General atomic and molecular electronic structure system. J. Comput. Chem., 14(11), 1347–1363.
Reed, A. E., Curtiss, L. A., & Weinhold, F. (1988). Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 88(6), 899–926.
Badenhoop, J. K., & Weinhold, F. (1997). Natural bond orbital analysis of steric interactions. J. Chem. Phys., 107(14), 5406–5421.
Gledening, E. D., Badenhoop, J. K., Reed, A. E., Carpenter, J. E., & Weinhold, F. NBO 4.M. (1999). [Computer software]. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI.
Mullay, J. (1984). Atomic and group electronegativities. J. Am. Chem. Soc., 106(20), 5842–5847.
Epiotis, N. D., Cherry, W. R., Shaik, S., Yates, R., & Bernardi, F. (1977). Structural Theory of Organic Chemistry. Top. Curr. Chem., 70, 502 p.
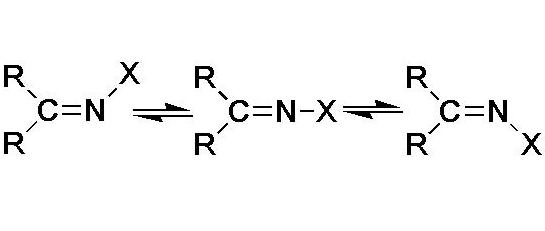
Downloads
Published
Issue
Section
License
Copyright (c) 2015 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

