Structure and redox properties of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) adsorbed on a silica surface. M05 computational study
DOI:
https://doi.org/10.15421/081511Keywords:
silica, adsorption, reduction, oxidation, CL-20Abstract
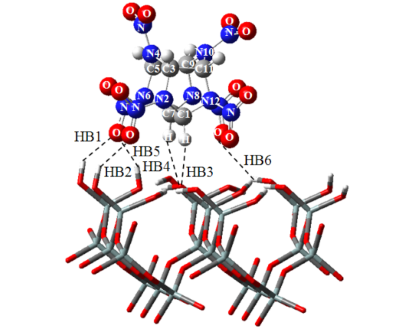
The cluster approximation was applied at M05/tzvp level to model adsorption of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20) on (001) surface of α-quartz. Structures of the obtained CL-20–silica complexes confirm close to parallel orientation of the nitrocompound toward surface. The binding between CL-20 and silica surface was analyzed and bond energies were calculated applying the atoms in molecules (AIM) method. Hydrogen bonds were found to significantly contribute in adsorption energy. An attaching of electron leads to significant deviation from coplanarity in complexes and to strengthening of hydrogen bonding. Redox properties of adsorbed CL-20 were compared with those of gas-phase and hydrated species by calculation of electron affinity, ionization potential, reduction Gibbs free energy, oxidation Gibbs free energy, reduction and oxidation potentials. It was shown that adsorbed CL-20 has lower ability to redox transformation as compared with hydrated one.References
Sviatenko, L. K., Isayev, O., Gorb, L., Hill, F. C., Leszczynska, D., & Leszczynski, J. (2015). Are the reduction and oxidation properties of nitrocompounds dissolved in water different from those produced when adsorbed on a silica surface? An DFT M05-2X computational study. J. Comput. Chem., 36(14), 1029–1035.
Sviatenko, L. K., Gorb, L., Hill, F. C., Leszczynska, D., & Leszczynski, J. (2015). ANTA structure and redox properties of 5-amino-3-nitro-1H-1,2,4-triazole (ANTA) adsorbed on a silica surface: A DFT M05 computational study. J. Phys. Chem. A., 119(29), 8139–8145.
Robidoux, P. Y., Sunahara, G. I., Savard, K., Berthelot, Y., Dodard, S., Martel, M., Gong, P., & Hawari, J. (2004). Acute and chronic toxicity of the new explosive CL-20 to the earthworm (Eisenia andrei) exposed to amended natural soils. Environ. Toxicol. Chem., 23, 1026–1034.
Kuperman, R. G., Checkai, R. T., & Simini, M. (2006). Environmental Fate and Transport of a New Energetic Material CL-20. U.S. Army Edgewood Chemical Biological Center, SERDP Report ER-1254.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G. A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H. P., Izmaylov, A. F., Bloino, J., Zheng, G., Sonnenberg, J. L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr. J. A., Peralta, J. E., Ogliaro, F., Bearpark, M., Heyd, J. J., Brothers, E., Kudin, K. N., Staroverov, V. N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J. C., Iyengar, S. S., Tomasi, J., Cossi, M., Rega, N., Millam, J. M., Klene, M., Knox, J. E., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Martin, R. L., Morokuma, K., Zakrzewski, V. G., Voth, G. A., Salvador, P., Dannenberg, J. J., Dapprich, S., Daniels, A. D., Farkas, Ö., Foresman, J. B., Ortiz, J. V., Cioslowski, J., & Fox, D. J. (2009). Gaussian 09 (Revision A.02) [Computer software]. Gaussian Inc., Wallingford CT.
Head-Gordon, M., Pople, J. A., & Frisch, M. J. (1988). MP2 energy evaluation by direct methods. Chem. Phys. Lett., 153, 503–506.
Weigend, F., & Ahlrichs, R. (2005). Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys., 7, 3297–3305.
Sviatenko, L., Isayev, O., Gorb, L., Hill, F., & Leszczynski, J. (2011). Toward robust computational electrochemical predicting the environmental fate of organic pollutants. J. Comput. Chem., 32, 2195–2203.
Kihara, K. (1990). An X-ray study of the temperature dependence of the quartz structure. Eur. J. Mineral., 2, 63–77.
Michalkova Scott, A., Burns, E. A., & Hill, F. C. (2014). Theoretical study of adsorption of nitrogen-containing environmental contaminants on kaolinite surfaces. J. Mol. Model., 20, 2373–2377.
Bader, R. W. F. (1990). Atoms in Molecules. A Quantum Theory. Oxford, UK: Calendon Press.
Lu, T., & Chen, F. (2012). Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem., 33(5), 580–592.
Cossi, M., Scalmani, G., Rega, N., & Barone, V. (2002). New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys., 117, 43–54.
Marenich, A. V., Cramer, C. J., & Truhlar, D. G. (2009). Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B, 113, 6378–6396.
Kholod, Y., Okovytyy, S., Kuramshina, G., Qasim, M., Gorb, L., & Leszczynski, J. (2007). An analysis of stable forms of CL-20: A DFT study of conformational transitions, infrared and Raman spectra. J. Mol. Struct., 843, 14–25.
Molt, R. W. Jr., Bartlett, R. J., Watson, T. Jr., & Bazante, A. P. (2012). Conformers of CL-20 Explosive and ab Initio Refinement Using Perturbation Theory: Implications to Detonation Mechanisms. J. Phys. Chem. A, 116, 12129−12135.
Mata, I., Alkorta, I., Molins, E., & Espinosa, E. (2010). Universal Features of the Electron Density Distribution in Hydrogen-Bonding Regions: A Comprehensive Study Involving H···X (X=H, C, N, O, F, S, Cl, p) Interactions. Chem. Eur. J., 16, 2442–2452.
Cremer, D., & Kraka, E. (1984). A description of the chemical bond in terms of local properties of electron density and energy. Croat. Chem. Acta, 57(6), 1259–1281.
Bader, R. F. W., & Essen, H. (1984). The characterization of atomic interactions. J. Chem. Phys., 80(5), 1943–1960.
Koch, U., & Popelier, P. L. A. (1995). Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem., 99, 9747–9754.
Tang, T.-H., Deretey, E., Knak Jensen, S. J., & Csizmadia, I. G. (2006). Hydrogen bonds: relation between lengths and electron densities at bond critical points. Eur. Phys. J. D, 37, 217–222.
Pourbaix, M. (1974). Atlas of Electrochemical Equilibria in Aqueous Solutions (2nd ed.). National Association of Corrosion Engineers: Houston.
Balakrishnan, V. K., Monteil-Rivera, F., Halasz, A., Corbeanu, A., & Hawari, J. (2004). Decomposition of the Polycyclic Nitramine Explosive, CL-20, by Fe0. Environ. Sci. Technol., 38, 6861–6866.
Uchimiya, M., Gorb, L., Isayev, O., Qasim, M. M., & Leszczynski, J. (2010). One-electron standard reduction potentials of nitroaromatic and cyclic nitramine explosives. Environ Pollut., 158, 3048–3053.
Downloads
Published
Issue
Section
License
Copyright (c) 2015 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

