FORMATION OF THE π-COMPLEXES OF COPPER ATOMS WITH ACRYLIC, MALEIC AND FUMARIC ACIDS IN AQUEOUS MEDIUM
DOI:
https://doi.org/10.15421/082016Keywords:
copper atoms, unsaturated organic acids, π-complexes, quantum-chemical modelingAbstract
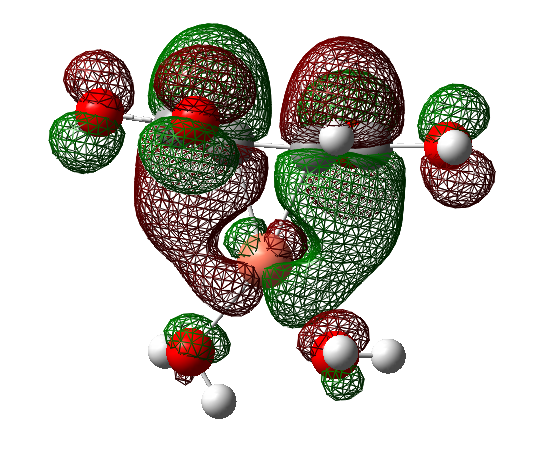
Using the methods of quantum chemical modeling, the interaction of copper atoms with acrylic (HA), maleic (H2M), and fumaric (H2F) acids in the presence of water has been studied. It was established that water molecules, being electron-donor ligands, strengthen the (dπ-pπ)-interaction in π-complexes of Cu0 with unsaturated organic acids. Eight stable structures with molecular forms of ligands (two with HA, three with H2M and H2F) were revealed, among which the most stable complexes are [Cu0(H2O)(HA)], [Cu0(H2O)2(H2M) and [Cu0(H2O)2(H2F)]. Topological analysis of the electron density distribution at the node Cu(-C=C-) showed that only in one case ([Cu0(H2O)2(H2F)]) copper atoms form molecular orbitals with both carbon atoms. In all other complexes, one carbon atom is involved in the formation of a π-bond. In addition, a synergistic effect of π-ligands on the binding energy of water molecules by copper atoms was found.
References
Vargalyuk, V. F., Polonsky, V. A., Orlenko, O.S. (2008). [Quantum-chemical study of the influence of olefinic compounds on the process of electrical reduction of copper ions]. Scientific Bulletin of Chernivtsi University. Series Chemistry, 399, 183–185. (in Ukrainian).
Vargalyuk, V. F., Polonsky, V. A., Orlenko, O.S., Vovk, R. S (2009). [Influence of acrylic acid on electrocrystallization of copper from sulfuric acid solutions]. Visnyk of Dnipropetrovsk University. Series Chemistry, 17(3/1), 35–38. (in Ukrainian).
Vargalyuk, V. F., Polonsky, V. A., Stets, O.S., Shchukin, A. I. (2011). [Influence of acrylic acid on the properties of copper coatings electrodeposited from sulfuric acid solutions]. Visnyk of Dnipropetrovsk University. Series Chemistry, 19(3/1), 13–17. (in Ukrainian).
Vargalyuk, V. F., Polonsky, V. A., Stets, O.S., Shchukin, A. I. (2012). [Infusion of acrylic acid on the power of medium coatings, electro-saggens and sulphate-acid solutions]. Bulletin of the Dnipropetrovsk University. Series Chemistry, 20(3/1), 15–12. (in Ukrainian).
Vargalyuk, V. F., Polonskyy, V. A., Stets, O. S., Shchukin, A. І. (2015). [Electrodeposition of copper in the presence of π-binding organic compounds]. Modern problems of electrochemistry, 234–235. (in Russian).
Iimura, T., Akasaka, N., Kosai, T., Iwamoto, T. (2017). A Pt(0) complex with cyclic (alkyl)(amino)silylene and 1,3-divinyl-1,1,3,3-tetramethyldisiloxane ligands: synthesis, molecular structure, and catalytic hydrosilylation activity. Dalton Transactions, 46(27), 8868–8874.http://dx.doi.org/10.1039/c7dt01113j
Braunschweig, H.,Arrowsmith, M., Celik, M. A., Dellermann, T., Dewhurst, R.D., Ewing, W.C., Hammond, K., Kramer, T., Krummenacher, I., Mies, J., Radacki, K., Schuster, J.K. (2016). Neutral zero-valent s-block complexes with strong multiple bonding. Nature chemistry, 8(9), 890–894.
http://dx.doi.org/10.1038/NCHEM.2542
Bauza, A., Frontera, A. (2018). Regium-π vscation-π interactions in M2 and MCl (M = Cu, Ag and Au) complexes with small aromatic systems: an ab initio study. Inorganics, 6(3), 64. http://dx.doi.org/10.3390/inorganics6030064
Vargalyuk, V. F., Polonskyy, V. A., Kramska, O.S., Shchukin, A. І. (2016). [The influence of acrylonitrile on electrode processes with the participation of copper cations]. Bulletin of the Dnipropetrovsk University. Series: Chemistry, 23(2), 22–26. (in Russian).
https://doi.org/10.15421/081514
Jonas, K., Krüger, C. (1980). Alkali Metal‐Transition Metal π-Complexes. AngewandteChemie International Edition in English, 19(7), 520–537.
https://doi.org/10.1002/anie.198005201
Wang, R., Yang, S., Li, Q. (2019). Coinage-Metal Bond between [1.1.1]Propellane and M2/MCl/MCH3 (M = Cu, Ag, and Au). Cooperativity and Substituents. Molecules, 24(14), 2601.
http://dx.doi.org/10.3390/molecules24142601
Wang, G., Freeman, L. A., Dickie, D. A., Mokrai, R., Benko, Z., GilliardJr, R. J. (2019). Isolation of Cyclic (Alkyl)(Amino) Carbene–Bismuthinidene Mediated by a Beryllium(0) Complex. Chemistry–A European Journal, 25(17), 4335–4339.
http://dx.doi.org/10.1002/chem.201900458
Beillard, A., Metro, T. X., Bantreil, X., Martinez, J., Lamaty, F. (2017). Cu(0), O2 and mechanical forces: A saving combination for efficient production of Cu–NHC complexes. Chemical science, 8(2), 1086–1089. http://dx.doi.org/10.1039/c6sc03182j
Liu, B., Ma, X., Wu, F., Chen, W. (2015). Simple synthesis of neutral and cationic Cu-NHC complexes. Dalton Transactions, 44(4), 1836–1844.
http://dx.doi.org/10.1039/x0xx00000x
Vargalyuk, V.F., Polonskyy, V.A., Stets, O.S., Balalaev, O. K. (2013). [Structure and properties of copper coatings electrodeposited from sulfuric acid solutions containing acrylic acid and acrylamide]. Ukrainian Chemical Journal, 29(3), 51–58. (in Ukrainian).
Frisch, M. J. E. A., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Nakatsuji, H. (2016). Gaussian, Inc., Wallingford CT.
Wachters, A. J. (1970). Gaussian basis set for molecular wavefunctions containing third‐row atoms. The Journal of Chemical Physics, 52(3),
–1036.https://doi.org/10.1063/1.1673095
Becke, A. D. (1993). Density-Functional Thermochemistry. III. The Role of Exact Exchange. Indian Journal of Pure & Applied Physics,98(7),5648–5656.https://doi.org/10.1063/1.464913
Lee, C., Yang, W., Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical review B,37(2), 785.
https://doi.org/10.1103/PhysRevB.37.785
Barone, V., Cossi, M., Tomasi, J. (1998). Geometry optimization of molecular structures in solution by the polarizable continuum model. Journal of Computational Chemistry, 19(4), 404–417.
https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Tomasi, J., Mennucci, B., Cammi, R. (2005). Quantum mechanical continuum solvation models. Chemicalreviews, 105(8), 2999–3094.https://doi.org/10.1021/cr9904009
Biegler-König, F. B., Schönbohm, J., Bayles, D. (2001). AIM2000-a program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Karaush, N. N., Baryshnikov, G. V., Minaeva, V. A., Minaev, B. F. (2015). A DFT and QTAIM study of the novel d-block metal complexes with tetraoxa [8] circulene-based ligands. New Journal of Chemistry,39(10), 7815–7821.
https://doi.org/10.1039/C5NJ01255D
Espinosa, E., Molins, E., Lecomte, C. (1998). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters, 285(3/4), 170–173.https://doi.org/10.1016/S0009-2614(98)00036-0
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A., Glushkov, V. N. (2019). Features of (dπ-pπ)-binding of Cu(I) ions with acrylic, maleic and fumaric acids in aqueous solution. Journal of Chemistry and Technologies, 27(2), 148–157.
Downloads
Published
Issue
Section
License
Copyright (c) 2020 Днипровский национальный университет имени Олеся Гончара

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

