5-ARYL-7,8,9,10-TETRAHYDRO-5H-TETRAZOLO[1,5-а]THIOPYRANO[3,2-d]PYRIMIDINE 6,6-DIOXIDES – A NEW HETEROCYCLIC ENSEMBLE via MCR APPROACH
DOI:
https://doi.org/10.15421/jchemtech.v31i2.126304Keywords:
azaheterocycles, sulfones, 5-aminotetrazole, microwave irradiation, multicomponent reactions (MCR)Abstract
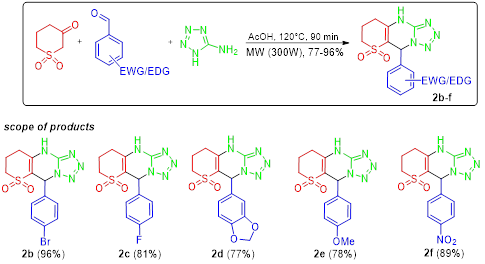
Tetrazoles are found broad applications in numerous fields such as in medicine, biochemistry, pharmacology, and in industry. Considering our continued interest in new azaheterocycles based on β-ketosulfones in this work a three-component heterocyclization of dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide, 1H-tetrazol-5-amine, and aromatic aldehydes under microwave irradiation was studied. Regardless of the reaction conditions, 5-aryl-7,8,9,10-tetrahydro-5H-tetrazolo[1,5-a]thiopyrano[3,2-d]pyrimidine 6,6-dioxides were isolated as sole reaction products in good to excellent yields. To the best of our knowledge mentioned azaheterocycles are novel and previously not reported heterocyclic ensemble. Proposed structures were confirmed by spectral methods. Considering druglikeness we can conclude that compounds match parameters for Lipinski, Ghose, Veber, Egan and Muegge rules, and also correspond to the V class of acute toxicity. In silico screening of the biological profile of new derivatives showed adequate ADMET properties along with high (60 % and more) probability levels of activity against such pathogens/diseases as Candida albicans, Alphis gossypii, Tripomastigote Chagas, Tcruzi amastigota, Tcruzi epimastigota etc.
References
Neochoritis, C. G., Zhao, T., Dömling, A. (2019). Tetrazoles via multicomponent reactions. Chem. Rev., 119, 1970–2042. https://doi.org/10.1021/acs.chemrev.8b00564
Pokhodylo, N. T., Tupychak, M. A., Palchykov, V. A. (2020). Dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide – a new cyclic ketomethylenic reagent for the Dimroth-type 1,2,3-triazole synthesis. Synth. Commun., 50, 1835–1844. https://doi.org/10.1080/00397911.2020.1757113
Chabanenko, R. M., Mykolenko, S. Yu., Kozirev, E. K., Palchykov, V. A. (2018). Multigram scale synthesis of 3,4- and 3,6-dihydro-2H-thiopyran 1,1-dioxides and features of their NMR spectral behavior. Synth. Commun., 48, 2198–2205. https://doi.org/10.1080/00397911.2018.1486427
Palchikov, V. A., Gaponov, A. A., Chabanenko, R. M., Mykolenko, S. Yu. (2018). Synthesis of a New Spiro System: 1-Oxa-7-thia-4-azaspiro[4.5]decane 7,7-Dioxide. Russ. J. Org. Chem., 54, 588–592. https://doi.org/10.1134/S1070428018040127
Palchykov, V. A., Chabanenko, R. M., Konshin, V. V., Dotsenko, V. V., Krivokolysko, S. G., Chigorina, E. A., Horak, Y. I., Lytvyn, R. Z., Vakhula, A. A., Obushak, M. D., Mazepa, A. V. (2018). Dihydro-2H-thiopyran-3(4H)-one-1,1-dioxide – a versatile building block for the synthesis of new thiopyran-based heterocyclic systems. New J. Chem., 42, 1403–1412. https://doi.org/10.1039/C7NJ03846A
Kolomoets, O. S., Voskoboynik, O. Yu., Antypenko, O. M., Berest, G. G., Nosulenko, I. S., Palchikov, V. A., Karpenko, O. S., Kovalenko, S. I. (2017). Design synthesis and Anti-inflammatory Activity of Derivatives 10-R-3-Aryl-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones of Spiro-fused Cyclic Frameworks. Acta Chim. Slov., 64, 902–910. https://doi.org/10.17344/acsi.2017.3575
Voskoboynik, O. Yu., Kolomoets, O. S., Palchikov, V. A., Kovalenko, S. I., Belenichev, I. F., Shishkina, S. V. (2017). [1,2,4]Triazino[2,3-с]quinazolines 2*. Synthesis, structure, and anticonvulsant activity of new 3'-R1-spiro[(aza/oxa/thia)cycloalkyl1(3,4),6'-[1,2,4]triazino[2,3-c]quinazolin]-2'(7'H)-ones. Chem. Het. Comp., 53, 1134–1147. https://doi.org/10.1007/s10593-017-2184-8
Mohlala, R. L., Rashamuse, T. J., Coyanis, E. M. (2023). Multicomponent reactions as an efficient and facile alternative route in organic synthesis and applications. Preprints. Org. 2023060345. https://doi.org/10.20944/preprints202306.0345.v1
Vishwakarma, R., Chandrakanth, G., Lakshmi, K. M. (2022) Advances in Tetrazole Synthesis – An Overview. Chem. Select, 7, e202200706. https://doi.org/10.1002/slct.202200706
John, E. S., Gulatia, Sh., Shankaraiah, N. (2021). Recent advances in multi-component reactions and their mechanistic insights: a triennium review. Org. Chem. Front., 8, 4237–4287. https://doi.org/10.1039/D0QO01480J
Kozirev, E. K., Palchykov, V. A. (2019). Thiopyran-3-one 1,1-dioxides in the synthesis of heterocycles. Chem. Heter. Comp. 55, 349–351. https://doi.org/10.1007/s10593-019-02463-z
Kour, P., Singh, V. P., Khajuria, B., Singh, T., Kumar, A. (2017). Al(III) chloride catalyzed multi-component domino strategy: synthesis of library of dihydrotetrazolo[1,5-a]pyrimidines and tetrahydrotetrazolo[1,5-a]quinazolinones. Tetrahedron Letters., 58, 4179–4185. https://doi.org/10.1016/j.tetlet.2017.09.052
Wang, X-S., Yang, K., Zhou, J., Tu, S-J. (2010). Facile Method for the Combinatorial Synthesis of 2,2-Disubstituted Quinazolin-4(1H)-one Derivatives Catalyzed by Iodine in Ionic Liquids. J. Comb. Chem. 12, 35–40. https://doi.org/10.1021/cc900174p
Shaik, F. B., Nagendra, T. P., Babu, V. G., Shanthi, V. K., Mulakayala, N., Anwar, Sh. (2019). An efficient, multicomponent, green protocol to access 4,7-dihydrotetrazolo[1,5-a]pyrimidines and 5,6,7,9-tetrahydrotetrazolo[5,1-b]quinazolin-8(4H)-ones using PEG-400 under microwave irradiation. Synth. Comm., 49, 3181–3190. https://doi.org/10.1080/00397911.2019.1659973
Antoine D., Olivier, M., Vincent, Z. (2017). SwissADME: a free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7, 42717 https://doi.org/10.1038/srep42717
Banerjee, P., Andreas, O. E., Schrey, A. K., Preissner, R. (2018). ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Research, 46, W257–W263 https://doi.org/10.1093/nar/gky318
Scotti, M. T., Herrera-Acevedo, C., de Menezes, R. P. B., Martin, H-J., Muratov, E. N., Silva, Á.Í.d.S., Albuquerque, E. F., Calado, L. F., Ericsson, C.-B., Scotti, L. (2022). MolPredictX: online biological activity predictions by machine learning models. Mol. Inf. 41(12), e2200133. https://doi.org/10.1002/minf.202200133
Lipinski, C. A., Lombardo, F., Dominy, B. W., Feeney P. J. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev., 46, 3–26. https://doi.org/10.1016/s0169-409x(00)00129-0
Ghose, A. K., Viswanadhan, V. N., Wendoloski, J. J. (1999). A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem., 1, 55–68. https://doi.org/10.1021/cc9800071
Veber, D. F., Johnson, S. R., Cheng, H-Y., Smith, B. R., Ward, K. W., Kopple, K. D. (2002). Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem., 45, 2615–2623. https://doi.org/10.1021/jm020017n
Egan, W. J., Merz, K. M., Baldwin J. J. (2000). Prediction of drug absorption using multivariate statistics. J. Med. Chem., 43, 3867–3877. https://doi.org/10.1021/jm000292e
Muegge, I., Heald, S. L., Brittelli, D. (2001). Simple selection criteria for drug-like chemical matter. J. Med. Chem., 44, 1841–1846. https://doi.org/10.1021/jm015507e
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

