Electrochemical degradation of methyl tert-butyl ether
DOI:
https://doi.org/10.15421/081406Keywords:
methyl tert-butyl ether (MTBE), electrochemical oxidation, lead dioxide anode, degradation mechanismAbstract
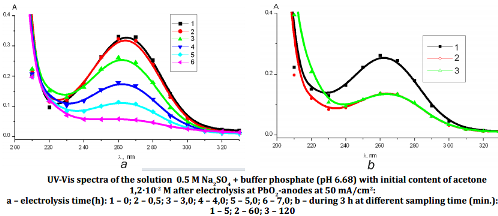
In this paper, we have examined the performance of PbO2 anodes in the EC degradation of MTBE. It was shown that electrochemical oxidation of MTBE at lead dioxide anodes is effective method of anodic conversion of the organic pollutant to acetic acid as untoxic product. Proposed method is formally reagent treatment of water at the same time it does not need addition of any reagent in reaction media. All needed reagents formed directly from the solvent (water) thanks to electrochemical reactions. According to obtained data the main electrochemical stages of the process of anodic conversion of MTBE are formation of hydroxyl-radicals and molecular oxygen. Then formed compounds take part in stages of chemical MTBE oxidation and intermediate species that led to deeper oxidation to form acetic acid as the result. Proposed mechanism of MTBE electrochemical oxidation is in satisfactory agreement with experimental data. Dependence of MTBE conversion rate from the nature of micro-doped and composite lead dioxide anodes is explained by difference in hydroxyl-radical bond strength with an electrode surface that determined it reaction ability in secondary chemical reactions of organic compounds oxidation.References
Froines J. Health and Environmental Assessment of MTBE-UC Toxics Sub-stances Research and Teaching Program, 1998, vol. 2, р.36.
Strategic Environemntal Research Development Program – SERDP Infor-mation Bulletin, 1999, vol. 1.
Mitani M.M., Keller A.A., Golden S.J., Hatfield A.K. Cheetham. Low tem-perature catalytic decomposition and oxidation of MTBE. Applied Catalysis B: Environ-mental, 2001, Vol.34, p. 87–95.
Graham J.L., Striebich R., Patterson C.L., Radha Krishman R.C. Haught. Haught MTBE oxidation byproducts from the treatment of surface waters by ozonation and UV-ozonation. Chemosphere, 2004, vol.54, p. 1011-1016.
Xiang-Rong Xu, Zhen-Ye Zhao, Xiao-Yan Li, Ji-Dong Gu. Chemical oxidative degradation of methyl tert-butyl ether in aqueous solution by Fenton’s reagent. Chemosphere, 2004, vol. 55, p. 73-79.
Kang J.W., Hoffmann M.R. Kinetics and Mechanism of the Sonolytic De-struction of Methyl tert-Butyl Ether by Ultrasonic Irradiation in the Presence of Ozone. Environ. Sci. Technol., 1998. vol.32, p. 3194-3199.
Wu T., Cruz V., Mezyk S., Cooper W.J., O’Shea K.E. Gamma radiolysis of methyl t-butyl ether: a study of hydroxyl radical mediated reaction pathways. Radiation Physics and Chemistry, 2002, vol.65, p. 335-341.
Safarzadeh-Amiri A. O3/ H2O2 tretment of Methyl tert-Butyl Ether (MTBE) in contaminated waters. Wat. Res., 2001, vol.35, p. 3706-3714.
Sutherland J., Adams C., Kekobad J. Fenton’s oxidation of MTBE with zero-valent iron. Wat. Res., 2004, vol.38, p. 193-205.
Bergendahl J.A., Thies T.P., Wat. Res., 2004, vol.38, p. 327-334.
Johnson D.C., Feng J., Houk L.L. Direct electrochemical degradation of or-ganic wastes in aqueous media. Electrochim. Acta., 2000, vol. 46, no. 2-3, p. 323-330.
Velichenko A.B. Mikromodifitsirovannyie dioksidnosvintsovyie elek-trodyi: Diss. … doktora him. nauk: 02.00.05. , Dnepropetrovsk, 2002, 337 s.
Obschaya organicheskaya himiya. Kislorodsoderzhaschie soedineniya, pod red. N.K. Kochetkova, A.I. Usova, M.: Himiya, 1982, vol. 2, 855 s.
Petriy O.A., Podlovchenko B. I., Elektrokataliticheskie protsessyi okisleniya i vosstanov-leniya organicheskih veschestv, v kn.: Kataliz. Fundamentalnyie i prikladnyie is-sledovaniya, M.: Izd-vo MGU ,1987, s. 39-64.
Rayd K., Kurs fizicheskoy organicheskoy himii, M.: Mir, 1972, s.353.
Todres Z.V. Ion-radikalyi v organicheskom sinteze , M.: Himiya, 1986, s. 43.
Organicheskaya elektrohimiya, pod red. Petrosyana V. A., Feoktistova L. G., M.: Himiya, 1988, vol. 2, 498 s.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

