The structures of complexes of Chromium(III) with cystine and ethylglycine
DOI:
https://doi.org/10.15421/081318Keywords:
chrom, complex connections, aminoacidesAbstract
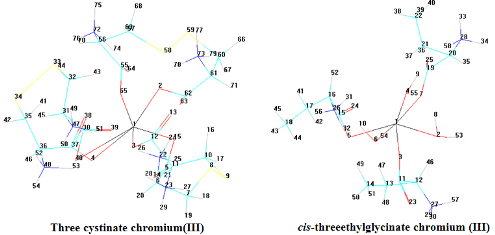
It has been investigated the electronic spectra of complex compounds of chromium(III) with amino acids as bidentate coordinated in compound (I) and as monodentate coordinated in compound (II) in solution. Three-ethyl glycinate and three-cystinate have the octahedral structure. Two bands of transition were observed in the visible part of the spectrum 4Т1g¬4A2g and 4Т2g¬4A2g, one band of transition was observed in UV- spectrum, 4Т1g(Р)¬4A2g. It was submitted the results of calculations of crystal field parameters: Dq = 1790 cm-1, В = 560.57 cm-1 and b = 0.54 for compound (I), Dq = 1786 cm-1, В = 504.53sm-1 and b = 0.49 for compound (II). The computer three-dimensional models of structure of such chromium(III) complexes as three-cystinate chromium (III) and three-chromium glycinate were created. Quantum-chemical modeling programs Chemcraft and WinGAMESS were used. Angles and bond lengths were calculated.
References
Babenko, G. A. Biologicheskaya rol mikroelementov i ih primenenie v selskom hozyaystve i meditsine, Moskow: Meditsina, 1974, 283 p.
Soroka, V. R., Geutskaya, G. I., Valyichevtseva, L. G. Correction of the biochemical changes and ion-bound chromium complex with allacsane diabetes. Endokrinologiya. Respubl. mezhved. Sbornik, 1989, p. 45-49.
Chernushenko, E. A, Vinichenko, I. G. Hypoglycemic activity of the compounds of chromium. Visn. Dnipropetr. univ.: Meditsina i ohorona zdorov’ya, 2003, no. 4, p. 117-120.
Drago, R. Fizicheskie metodyi v neorganicheskoy himii, Moskow: Mir, 1967, 448 p.
Bellami, L. Infrakrasnyie spektryi slozhnyih molekul, Moskow: Mir, 1963, 337 p.
Downloads
Published
Issue
Section
License
Copyright (c) 2014 Vìsnik Dnìpropetrovsʹkogo unìversitetu. Serìâ Hìmìâ

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

