Electrode-analytical properties of polyvinylchloride membranes based on triple metal-polymeric complexes
DOI:
https://doi.org/10.15421/081506Keywords:
triple metal-polymeric complex, polyvinylpyrrolidone, apple juice, beer, wine, brandyAbstract
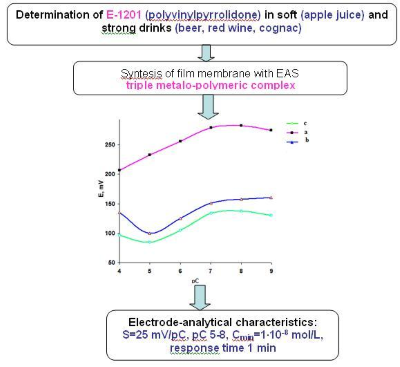
The influence of the nature of the electrode-active substances (EAS), the composition of the external and internal solutions on the formation of the analytical signal of polyvinylchloride (PVC) membranes based on associates and triple metal-polymeric complexes (TMPC) was established. Dehumidification of synthesized membranes increases with the content of polyvinylpyrrolidone (PVP). The value of the swelling degree is more than two times greater for membranes, which contain as EAS TMPC, relative to membranes based on associates. The value of water absorption of membranes is determined by the nature of EAS. They formed a series of increasing of the swelling degree such as associate < background membrane < TMPC. Swelling of the background membrane is explained by the physical sorption of water molecules on the surface of plasticized membrane. Hydration of PVP macromolecules varies with the introduction of metal ions, macromolecules unit undergoes a conformational transition. PVP macromolecules form tunnels or cavities where complex particles distributed and additional water accumulated through the second coordination layer. Constructed sensors based on TMPC have slope of electrode function equal to 25 mV/pC. Linear dependence of potential on the polymer concentration is observed in the range of 5–7 pC units. Sensors based on associates have slope of the electrode function of 20–25 mV/pC that can be varied depending on the nature of the EAS. Working range is 4–8 pC. Response time of sensor is less than 1 min. The optimal time for conditioning of the synthesized PVC membrane is 24 hours. Potentiometric sensors have been developed for the determination of residual amounts of low molecular PVP which is a food additive E 1201 commonly used for thickening, stabilizing and clarifying of food products. The content of PVP was determined in real objects (apple juice, beer, red wine and cognac) with using the polyvinylpyrrolidone sensors (Sr < 0.08). The advantages of the proposed technique of direct potentiometric determination of food additives E-1201 are following: the low limit of detection (1·10‒8 M), rapidity (2–5 min) and the absence of complex stages of sample preparation. Developed potentiometric sensors can be used in laboratories for food control, in particular, alcoholic and soft drinks, the content of the food additive such as E-1201.References
Korenman, Ya. I., & Kuchmenko, T. A. (2002). [Approaches for food products analyses. Development of mass-sensitive sensors]. Rosiyskiy Khimicheskiy Zhurnal – Russian Chemical Journal, 46(4), 34–42 (in Russian).
Tovstenko, Yu. V., Derkach, T. M., & Tkach, V. I. (2008). [Analytical monitoring of oxytetracycline hydrochloride content in milk products by electrochemical methods]. Metody i obekty khymicheskoho analyza – Methods and objects of chemical analysis, 3(2)191–201 (in Ukrainian).
Pashyna, O. V., & Tkach, V. I. (2012). [Determination of syntetic sweetener aspartame (food addition Е-951) by direct potentiometry]. Metody i obekty khymicheskoho analyza – Methods and objects of chemical analysis, 7(13), 143–152 (in Ukrainian).
Tkach, V. I., Maha, I. M., & Bolotyn, O. V. (2012). [Electrochemical analysis of nitrogen containing organic pharmaceutical and bioactive substances with the using heteropolyanions of Keggin structure as analytical reagents]. Uzhgorod, Ukraine: Publishing of V. Podiaka (in Ukrainian).
Panasyuk, N., Tkachenko, Ya., & Tkach, V. (2012). [Using of heteropolyacids of Keggin structure for the ionometric determination of riboflavin]. Visn. L'viv. Univer.: Khim. – Bull. Lviv. Univer.: Chem., (53), 208–215 (in Ukrainian).
Chmilenko, F. A., Korobova, V. I., & Mikulenko, O. V. (2008). [Potentiometric sensors for determination of water-soluable polyelectrolities]. J. Analyt. Chem., 63(6), 645–650.
Chmilenko, F. A., Коrobova, I. V., Gurtovaya, O. V., & Chmilenko, T. S. (2009). [Potentiometric membrane sensors for polyvinylpyrrolidone determination]. Talanta, 78(4–5), 1259–1265.
Chmilenko, T. S., Matorina, K. V., & Chmilenko, F. A. (2013). [Potentiometric sensors for determination of high-molecular polyvinylpyrrolidone]. Metody i obekty khymicheskoho analyza – Methods and objects of chemical analysis, 8(2), 63–71 (in Russian).
Chmilenko, F. O., Matorina, K. V., Korobova, I. V., & Chmilenko, T. S. (2009). [Ionometric determination of higher molecular fractions of polyvinylpyrrolidone]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (2), 91–95 (in Ukrainian).
Chmilenko, T. S., Matorina, K. V., & Chmilenko, F. A. (2011). [Sensors with potentiometric registration bases on associates and triple metal-polymeric complexes with polyvinylpyrrolidone as electrode-active substances of membranes]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (4/2), 277–279 (in Russian).
Chmilenko, T. S., Matorina, K. V., & Chmilenko, F. A. (2011). [Triple metal-polymeric complexes as electrode-active components of plastificated membranes of ion-selective electrode]. Visn. Dnipropetr. Univer.: Khim. – Bull. Dnipropetr. Univer.: Chem. 19(17), 129–135 (in Ukrainian).
Kharitonov, S. V. (2007). Ion–selective electrodes in medicinal drug determination. Russ. Chem. Rev., 76(4), 361–396.
Mikhel'son, K. N. (2008). Electrochemical sensors based on ionophores: Current state, trends, and prospects. Rus. J. Gen. Chem., 78(12), 2445–2454.
Chmilenko, T. S., Matorina, K. V., & Burmistrov, K. S. (2012). [Spectrophotometric determination of polyvinylpyrrolidone and polyvinyl alcohol with the help of cationic dyes]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (5), 119–124 (in Ukrainian).
Yu, L., Liu, Z., Hu, X., Kong, L., & Liu, S. (2010). Fluorescence quenching reaction of polyvinylpyrrolidone-eosin Y system for the determination of polyvinylpyrrolidone. J. Fluoresc., 20, 733–738.
Chadna, R., Kapoor, V. K., & Kumar, A. (2006). Analytical techniques used to characterize drug – polyvinyl-pyrrolidone systems in solid and liquid states. J. Scientific & Industrial Research, 65, 459–469.
Chmilenko, F. O., Zhuk, L. P., Chmilenko, T. S., Mikulenko, O. V., & Tereshenko, O. V. (2005). [Water-soluable polymers as analytical reagents]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (6), 31–42 (in Russian).
Lihong, Y. U., Zhongfang, L. I. U., & Shaopu, L. I. U. (2009). Fading Spectrophotometric Method for the Determination of Polyvinylpyrrolidone with Eosin Y. Chinese J. Chem., 27(8), 1505–1509.
Sadao, M. (1983). Calibration of size exclusion chromatography columns for molecular weight determination of polyacrylonitrile and PVP in N,N-dimethylformamide. Anal. Chem., 55(14), 2414–2416.
Chmilenko, F. O., Mikulenko, O. V., Chmilenko, T. S., & Bilchuk, V. S. (2005). [Polyvinylpyrrolidone spaces distribution for molecular mass determinated by high liquid chromato-graphy]. Voprosy khimii i khimicheskoi technologii – Issues of Chemistry and Chemical Technology, (4), 12–15 (in Russian).
Beneito-Cambra, M., Herrero-Martínez, J. M., & Ramis-Ramos, G. (2009). Characterization and determination of poly(vinylpyrrolidone) by complexation with an anionic azo-dye and nonequilibrium capillary electrophoresis. J. Chromatogr. A., 1216(51), 9014–9021.
Takagishi, T., Naoi, G., & Kuroki, N. (1977). Interaction of poly(vinylpirrolidone) with the hydrophobic fluorescent probe, 2-p-toluidinylaphthalene-6-sulfonate. J. Polym. Sci.: Polym. Chem. Ed., 15, 2789–2790.
Ovsepyan, A. M., Kobyakov, V. V., Dubrovin, V. I., & Panov, V. P. (1978). Determination of polyvinylpyrrolidone in aqueous solutions by IR-spectrophotometric and spectrofluorimetric methods. Pharm. Chem. J., 12(11), 1517–1520.
Antić, V. V., Antić, M. P., & Kronimus, A. V. (2011). Quantitative determination of poly(vinylpyrrolidone) by continuous-flow off-line pyrolysis-GC/MS. J. Analyt. Applied Pyrolysis, 90(2), 93–99.
Ericsson, I., & Ljunggren, L. (1990). Trace determination of high molecular weight polyvinylpyrrolidone by pyrolysis-gas chromatography. J. Analyt. Applied Pyrolysis, 17(3), 251–260.
Tavlarakis, P., Urban, J. J., & Snow, N. (2010). Determination of Total Polyvinylpyrrolidone (PVP) in Ophthalmic Solutions by Size Exclusion Chromatography with Ultraviolet-visible Detection. J. Chromatogr. Sci., 49(6), 457–462.
Sidelkovskaya, F. A. (1970). [Chemistry of polyvinylpyrrolidone and its polymers]. Moskow, USSR: Nauka (in Russian).
Skripchuk, V. G., & Kozubovskiy, A. I. (1987). [Methods of water soluable synthetic polymers – polyacrylamide, polyvinyl alcohol and polyvinylpyrrolidone]. J. Analyt. Chem., 42(3), 389–397.
Kirsh, U. E. (1998). [Poly–N-vinylpyrrolidone and other poly-N-vinylamides]. Moskow, Russian Federation: Nauka (in Russian).
Verdier, E., Piro, J., & Montelongo, F. (1971). Quantitative definition of polyvinylpirrolidone by the method of electric adsorption. Talanta, 18(12), 1237–1241.
Chmilenko, F. O., Matorina, K. V., Chmilenko, T. S. (2009). [Express – control of physiological – active polymer polyvinylpirrolidone contain with perspective to using in space conditions]. Ecologia i noospherologia – Ecology and Noospherology, 20(1–2), 71–77. (in Ukrainian).
Zorin, I., Scherbinina, T., & Fetin, P. (2014). Novel surfactant-selective membrane electrode based on polyelectrolyte–surfactant complex. Talanta, 130(1), 177–181.
Suberlyak O. V, Melnik Yu. Ya., & Baran N. M. (2006). [Polyamide membranes modificated by polyvinyl- pirrolidone]. Nauk. Zapyski NaUKMA «Khimichni nauki I tekhnologiy» – Science Notes NaUKMA «Chemical Sciences and Technology», 55, 19–23 (in Ukrainian).
Kirsh, Yu. E., Smirnov, S. A., Popkov, Yu. M., & Timashev, S. F. (1990). Perfluorinated carbon–chain copolymers with functional groups and cation exchange membranes based on them: synthesis, structure and properties. Russ. Chem. Rev., 59(6), 560–574.
Gerasimenko, K. O., Chervakov, O. V., & Kobelchuk, U. M. (2009). [Film sulfonic acid polyamides reinforced by microporous separators]. Voprosy khimii i khimicheskoi technologii –Issues of Chemistry and Chemical Technology, (2), 68–71. (in Russian).
Balasubramanian, D., & Mirsa, B. (1977). Metal-ligand interactions. Boston: Dordrecht.
Lyashchenko, A. K., Lileev, A. S., Pollitskaya, T. A., & Ostroushko, A. A. (2001). Dielectric Relaxation Characteristics of Water in Water–Polyvinyl Alcohol and Water–Polyvinylpyrrolidone Mixed Solvents. Russ. J. Phys. Chem. A., 75(2), 202–262.
Ostroushko, A. A., & Minyaev, V. I. (2003). Phase Relations in the Lanthanum Nitrate–Poly(vinyl Alcohol)–Water System. Russ. J. Inorg. Chem., 48(11), 1728–1731.
Pomogaylo, A. D., & Uhlyand, I. E. (1991). [Macromolecular metal–chelates]. Moskow, Russian Federation: Khimia (in Russian).
Ostroushko, A. A., Sennikov, M. Yu., & Gerasimova, E. L. (2005). Electrochemical and Electrophysical Parameters of Polymer–Salt Compositions Based on Poly(vinyl Alcohol) and Ammonium Heptamolybdate. Russ. J. Inorg. Chem., 50(3), 428–433.
Fernandes, Sh., Kim, H.–S., & Hatti-Kaul, R. (2002). Affinity extraction of dye- and metal ion-binding proteins in polyvinylpyrrolidone-based aqueous two-phase system. Protein Express. Rurific., 24(3), 460–469.
Sadeghi, R. (2005). Vapor–liquid equilibria of the polyvinylpyrrolidone + (NH4)2SO4 +H2O msystem at different temperatures. Fluid Ph. Equil., 233(2), 176–183.
Sadeghi, R. (2005) Measurement and correlation of phase equilibria for several PVP + salt aqueous two-phase systems at 303.15K. Fluid Ph. Equil., 237(1–2), 40–47.
Downloads
Published
Issue
Section
License
Copyright (c) 2015 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

