STUDY OF THE STRUCTURE AND THERMAL PROPERTIES OF IRON-CONTAINING COMPOSITES BASED ON PALYGORSKITE FOR THE EXTRACTION OF Cr(VI) AND U(VI) IONS
DOI:
https://doi.org/10.15421/jchemtech.v30i4.262446Keywords:
palygorskite; nZVI; microscopic examinations; XRD; thermal analysis; adsorption; chromium(VI); uranium(VI).Abstract
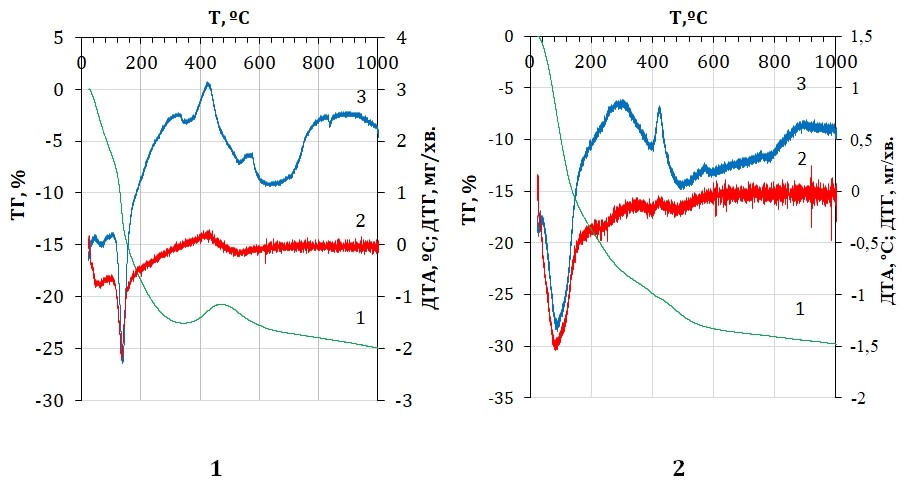
Aim. Study of the structure, thermal and sorption properties of iron-containing sorbent based on palygorskite. Methods. To study the structure of the composite, X-ray diffraction analysis and microscopic studies were used. Thermal analysis was carried out in order to refine the structure of materials and to establish temperature intervals for drying and utilizing sorbents. Sorption properties of materials determined by spectrophotometric method. Results. X-ray diffraction of the obtained material confirmed that particles of nano-sized zero-valent iron were fixed on the surface of palyhorskite. Transmission electron microscopy of the samples confirmed that nanoscale zero-valent iron forms spherical particles arranged in the form of aggregates and chains. The resulting composite is characterized by a uniform distribution of nanosized zero-valent iron particles on the surface of palyhorskite. The thermal analysis of the iron-containing composite based on palyhorskite confirmed that only physically bound water is released up to 170 °С, which allows us to justify the maximum drying temperature of the material. The structural transformation of the mineral begins at a temperature above 960 °С. Under these conditions, new phases are formed, which confirms the possibility of recycling spent sorbents using ceramic technology. Exothermic effects corresponding to iron oxidation temperatures in sorbents allow us to state that the size of iron particles in an iron-containing composite is smaller than the size of nano-sized zero-valent iron obtained from a solution of ferrum(II) sulfate. These data also confirm the results of X-ray diffraction. Sorption experiments confirmed that the synthesized composite effectively removes Cr(VI) and U(VI) from aqueous environments at pH values close to natural waters. An analysis of the results of sorption studies of samples dried at temperatures from 60 to 100 °C showed that the optimal temperature for drying an iron-containing composite based on palygorskite, under otherwise equal synthesis conditions, is 80 °C. Conclusions. The structure of the iron-containing composite has been studied. The maximum drying temperature of the synthesized sorbents and the possibility of recycling spent sorbents using ceramic technology have been established.
References
Dvoretskyi, A.I., Liashenko, V.I., Topolnyi, F.F., Kovalenko, H.D. (2018). [Influence of uranium industry on the state of environment and population]. Metalurhiina ta hirnychorudna promyslovist – Metallurgical and Mining Industry, 4, 99–109 (in Ukrainian). doi:10.33101/S04-4567678
Selvakumar, R., Ramadoss, G., Menon, M., Rajendran, K., Thavamani, P., Naidu, R., Megharaj, M. (2018). Challenges and complexities in remediation of uranium contaminated soils: A review. J. Eniron. Radioact. 192. 592–603. doi: 10.1016/j.jenvrad.2018.02.018
Yin, M., Sun, J., Chen, Y., Wang, J., Shang, J., Belshaw, N., Shen, C., Liu, J., Li, H., Linghu, W., Xiao, T., Dong, X., Song, G., Xiao, E., Chen, D. (2019). Mechanism of uranium release from uranium milltailingsun der long-termexposuret osimulate da cidrain: Geochemical evidence and environmental implication. Environ. Pollut. 244. 174–181. https://doi.org/10.1016/j.envpol.
10.018
Krajňák, A., Viglašová, E., Galamboš, M., Krivosudský, L. (2017). Application of HDTMA-intercalated bentonites in water waste treatment for U(VI) removal. J. Radioanal. Nucl. Chem. 314. 2489–2499. doi:10.1007/s10967-017-5590-6
Krajňák, A., Viglašová, E., Galamboš, M., Krivosudský, L. Krajňák, A., Viglašová, E., Galamboš, M., Krivosudský, L. (2018). Kinetics, thermodynamics and isothermparameters of uranium(VI) adsorption on natural and HDTMA-intercalated bentonite and zeolite. Dsalin. Water Treat. 127. 272–281. doi: 10.5004/dwt.2018.22762
Chen, A., Shang, C., Shao, J., Zhang, J., Huang, H. (2017) The application of iron-based technologies in uranium remediation: A review. Sci. Total. Environ. 575. 1291–1306. doi: 10.1016/j.scitotenv.2016.09.211
Zhdanyuk, N. (2017). Mechanism of the reduction of U (VI) by organoclay supported nZVI. Danish Scientific Journal. 4. 88–92.
Prus, V., Zhdanyuk, N. (2016). Investigation of removal of hexavalent chromium and divalent cobalt from aqueous solutions by organo-montmorillonite supported iron nanoparticles. EUREKA: Physics and Engineering. 5. 81–88. https://doi.org/10.21303/2461-4262.2016.00163
Jiang, D., Zeng, G., Huang, D., Chen, M., Zhang, C., Huang, C., Wan, J. (2018). Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 163. 217–227. doi: 10.1016/j.envres.2018.01.030
Ezzatahmadi, N., Ayoko, G., Millar, G., Speight, R., Yan, C., Li, J., Li, S., Zhu, J., Xi, Y. (2017). Clay-supported nanoscale zero-valent iron composite materials for the remediation of contaminated aqueous solutions: A review. Chem. Eng. J. 312. 336–350. doi: 10.1016/j.cej.2016.11.154
Yin, Y., Zheng, W., Yan, A., Zhang, C., Gou, Y., Shen, C. (2021). A Review on Montmorillonite-Supported Nanoscale Zerovalent Iron for Contaminant Removal from Water and Soil. Adsorption Science & Technology. Article ID 9340362, 19 pages. doi: https://doi.org/10.1155/2021/9340362
Doroshenko, D. V., Spasonova, L.M., Pavlenko, V.M., Kornilovych, B.Iu. (2017). [Reception of Nanostructured Materials Based on Illite for Toxic Substances Immobilization. Naukovi visti Natsionalnoho tekhnichnoho universytetu Ukrainy], "Kyivskyi politekhnichnyi instytut" - Research Bulletin of the National Technical University of Ukraine "Kyiv Politechnic Institute", 3(113). 95–103 (in Ukrainian). doi: 10.20535/1810-0546.2017.3.95151
Zhdanyuk, N. V. (2019). Development of sorbents based on modified clays to protect water from inorganic toxicants [Dissertation for the degree of a candidate of technical sciences in specialty 21.06.01 – «Environmental safety» (21 - National Security)]. https://er.nau.edu.ua/handle/NAU/39286
Pang, Z., Yan, M., X. Jia, Z. Wang, J. Chen (2014). Debromination of decabromodiphenyl ether by organomontmorillonite supported nanoscale zero-valent iron: Preparation, characterization and influence factors. J. Environ. Sci. 26. 483–491. doi:10.1016/s1001-0742(13)60419-2
Brindley, G.W., Brown, G. (1980). [Crystal structure of clay minerals and their X-ray identification: monograph]. London, UK: Miner. Soc.
Wells, A. (1988). [Structural inorganic chemistry: monograph]. Moscow, USSR: World (in Russian).
Zhdanyuk, N. V., Leshchenko, P.V. (2022). [Study of the structure, thermal and sorption properties of montmorillonite with a deposited layer of nano-sized zero-valent iron]. Naukovi visti Natsionalnoho tekhnichnoho universytetu Ukrainy "Kyivskyi politekhnichnyi instytut" - Academic notes of TNU named after V.I. Vernadskyi. Series: Technical sciences. 4(72). 230–235. (in Ukrainian). https://doi.org/10.32838/2663-5941/2022.4/34
Lurie, Yu.Yu. (1989). [Analytical chemistry of industrial wastewater: monograph]. Moscow, USSR: Chemistry (in Russian).
Lazarev, A.I. (1980). [Organic reagents in the analysis of metals: a handbook]. Moscow. USSR: Metallurgiya. (in Russian).
Korshunov, A.V. (2011). [Influence of dispersity of iron powders on the regularities of their oxidation when heated in air]. Zhurnal Yzvestyia Tomskoho polytekhnycheskoho unyversyteta - Journal of the Bulletin of the Tomsk Polytechnic University. 318(3), 5–11 (in Russian).
Zhdanyuk, N. (2017). Study of the structure of organo-modified palygorskite. Technology audit and production reserves. 5-3(37). 4–8. doi: 10.15587/2312-8372.2017.112900
Kubashevsky, O., Hopkins B. (1965). Oxidation of metals and alloys / translated from English. V. A. Alekseev. 2nd ed. Moscow, USSR: Metallurgiya (in Russian).
Ozovsky, A.Ya. (1974). Kinetics of topochemical reactions: monograph. Moscow, USSR: Chemistry, 1974 (in Russian).
Gubin, S. P., Koksharov, Yu. A., Khomutov, G. B., Yurkov, G. Yu. (2005). [Magnetic nanoparticles: production methods, structure and properties]. Uspekhy khymyy - Advances in Chemistry. 74(6). 539–574 (in Russian).
Ponmani, S., Udayasoorian, C. (2013). Zero Valent Iron (ZVI) nanocomposite for the removal of hexavalent chromium from aqueous solution. International Journal of Scientific and Engineering Research. 11, 588.
Filip, J., Karlický, F., Marusak, Z., Lazar, P., Cernik, M., Otyepka, M. Zbořil, R. (2014). Anaerobic Reaction of Nanoscale Zerovalent Iron with Water: Mechanism and Kinetics. J. Phys. Chem. C. 118. 13817−13825. https://doi.org/10.1021/jp501846f
Zhdanyuk, N. V., Kovalchuk, I.A., Kornilovych, B.Yu. (2018). [Sorption of uranium(VI) ions by ironcontaining nanocomposites based on montmorillonite]. Dopov. Nac. akad. nauk Ukr. - Reports of the National Academy of Sciences of Ukraine. 4. 88–93. (In Ukrainian).
https://doi.org/10.15407/dopovidi2018.04.088
Zhdanyuk, N. V., Kovalchuk, I.A., Kornilovych, B.Yu. (2019). [Sorption of uranium(VI) and cobalt(II) ions by iron-containing nanocomposites based on palygorskite]. Khimiia, fizyka ta tekhnolohiia poverkhni - Chemistry, physics and surface technology. 10(1), 48–58. (In Ukrainian). https://doi.org/10.15407/hftp10.01.048
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

