ISOLATION AND CHARACTERIZATION OF A PANCREATIC AMYLASE INHIBITOR
DOI:
https://doi.org/10.15421/jchemtech.v30i4.263201Keywords:
Key words: oat grain, oat flour; extraction; affinity chromatography; gel electrophoresis; pancreatic am-ylase inhibitor; molecular weight, biotechnology, pharmacy.Abstract
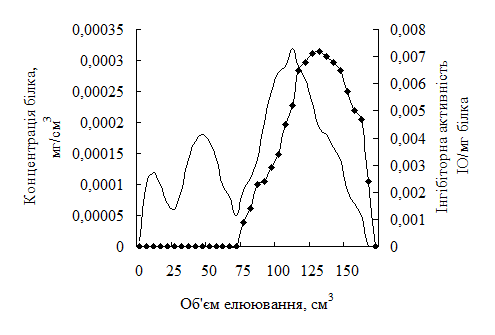
The role and sources of digestive enzyme inhibitors, which are effective correctors of digestive processes in the body, are characterized. Screening of vegetable raw materials zoned in Ukraine for the content of pancreatic α-amylase inhibitors showed the expediency of using secondary raw materials – oat flour Avena sativa. An inhibitor of pancreatic amylase from oat flour has been isolated and characterized. It has significant antiamylolytic activity. The obtained inhibitor is promising for creating compositions designed for nutritional correction of increased activation of amylolytic enzymes. Grain extracts and oat flour are characterized by significant inhibitory activity to pancreatic amylase. The water-soluble protein fractions from oat grains and oat flour have the maximum inhibitory activity. The highest degree of inhibitor extraction from oat flour occurs when extracting 0.15 M NaCl in 0.1 M hydrocarbonate buffer, pH 9.2. A monomeric pancreatic amylase inhibitor with a molecular mass of 25.11 kDa was isolated by affinity chromatography with a purity of 92.7; pH-optimum 5.5, thermo-optimum 37 0C. The inhibitor is most stable at pH 5.0 and 20 0C.
References
Celleno, L., Tolaini, M.V., D’Amore, A., Perricone, N.V., Preuss, H.G. (2007). A Dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and women. Int J Med Sci., 4(1), 45–52.
Guariguata, L., Whiting, D.R., Hambleton, I., Beagley, J., Linnenkamp, U., Shaw, J.E. (2014). Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice, 103(2):137–149. https://doi.org/10.1016/j.diabres.2013.11.002
Ulbricht, C., Bryan, J. K., Conquer, J., Costa, D., Stock, T., Tanguay-Colucci, S., Weissner, W. (2010). An Evidence-Based Systematic Review of Amylase Inhibitors by the Natural Standard Research Collaboration. Journal of Dietary Supplements, 7(1), 78–95.
https://doi.org/10.3109/19390210903535043
Krusir, G. V., Beltyukova, S. V., Liventsova O. O., Prylutsky V. P. (2019). [Isolation and physicochemical properties of tomato seed protease]. Pharmacol., 4:28-36. (in Ukrainian).
Krusir, G., Zakharchuk, V., Sevastyanova, E., Pylypenko, L., Moshtakov, S. (2020). Isolation of Alfalfa Seed Trypsin Inhibitor using Affinity Chromatography. Journal of Chemistry and Technologies, 28(3), 269–277. https://doi.org/10.15421/08202803
Tormo, M. A., Gil-Exojo, I., Romero de Tejada, A., Campillo, J. E. (2004). Hypoglycaemic and anorexigenic activities of an alpha-amylase inhibitor from white kidney beans (Phaseolus vulgaris) in Wistar rats. British Journal of Nutrition (Br J Nutr.), 92(5), 785–790. doi: 10.1079/bjn20041260
Bays, H. E. (2004). Current and investigational antiobesity agents and obesity therapeutic treatment targets. Obes Res., 12(8), 1197–1211. doi: 10.1038/oby.2004.151
Chokshi, D. (2007). Subchronic oral toxicity of a standardized white kidney bean (Phaseolus vulgaris) extract in rats. Food Chem Toxicol., 45(1), 32–40. doi: 10.1016/j.fct.2006.06.021
Reshta, S. P., Pylypenko, L. M., Danylova, O. I. (2021). [Physiological aspects of assessing the quality of grub products]. OLDI-PLYuS: 334. (in Ukrainian).
Arai, S., Yasuoka, A., Abe, K. (2008). Functional food science and food for specified health use policy in Japan: state of the art. Current Opinion in Lipidology, 19 (1), 69–73. doi: 10.1097/MOL.0b013e3282f3f505
Zoccatelli, G., Pellegrina, C. D., Mosconi, S., Consolini, M., Veneri, G., Chignola, R., Peruffo, A., Rizzi, C. (2007). Full-fledged proteomic analysis of bioactive wheat amylase inhibitors by a 3-D analytical technique: identification of new heterodimeric aggregation states. Electrophoresis., 28(3), 460–466. doi: 10.1002/elps.200600348
Iulek, J., Franco, O. L., Silva, M., Slivinski, C. T., Jr, B. C., Rigden, D. J., Grossi de Sá, M. F. (2000). Purification, biochemical characterisation and partial primary structure of a new α-amylase inhibitor from Secale cereale (rye). The International Journal of Biochemistry & Cell Biology, 32(11–12), 1195–1204.
Ninomiya, K., Ina, S., Hamada, A., Yamaguchi, Y., Akao, M., Shinmachi, F., Kumagai, H. and Kumagai, H. (2018). Suppressive Effect of the α-Amylase Inhibitor Albumin from Buckwheat (Fagopyrum esculentum Moench) on Postprandial Hyperglycaemia. Nutrients , 10(10), 1503; https://doi.org/10.3390/nu10101503
McCue, P.P., Shetty, K. (2004). Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac J Clin Nutr., 13(1), 101–106.
Svensson, B., Fukuda, K., Nielsen, P. K., Bønsager, B. C. (2004). Proteinaceous alpha-amylase inhibitors. Review. Biochim. Biophys. Acta, 1696(2), 145–156. doi: 10.1016/j.bbapap.2003.07.004.
Rekha, M. R., Padmaja, G. (2002). Alpha-amylase inhibitor changes during processing of sweet potato and taro tubers. Plant Foods Hum Nutr., 57(3–4), 285–294.
Cavalot, F., Petrelli, A., Traversa, M., Bonomo, K., Fiora, E., Conti, M., Anfossi, G., Costa, G., Trovati, M. (2006). Postprandial Blood Glucose is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. Journal of Clinical Endocrinology & Metabolism, 91(3), 813–819, https://doi.org/10.1210/jc.2005-1005
Morton, R. L., Schroeder, H. E., Bateman, K. S., Chrispeels, M. J., Armstrong, E., Higgins, T. J. (2000). Bean alpha-amylase inhibitor 1 in transgenic peas (Pisum sativum) provides complete protection from pea weevil (Bruchus pisorum) under field conditions. PNAS (Proc Natl Acad Sci U S A), 97(8), 3820–3825. https://doi.org/10.1073/pnas.070054597
Ostapchenko, L. I. (2012). [Biochemistry]. Kiev:NAU. (in Ukrainian).
Krusir, G., Zakharchuk, V., Sevastyanova, E., Pylypenko, L., Mazurenko, K. (2021). Isolation of lysozyme of Black sea mussel Mytilus galloprovincialis. Journal of Chemistry and Technologies, 29(3), 410–416. doi: 10.15421/jchemtech.v30i1.255480.
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

