QUANTUM CHEMICAL MODELING OF AQUACHLOROCOMPLEXES OF Cu+ WITH ACRYLIC, MALEIC AND FUMARIC ACIDS
DOI:
https://doi.org/10.15421/jchemtech.v30i4.263280Keywords:
acidoaquachlorocomplexes Cu , unsaturated organic acids, quantum chemical modeling.Abstract
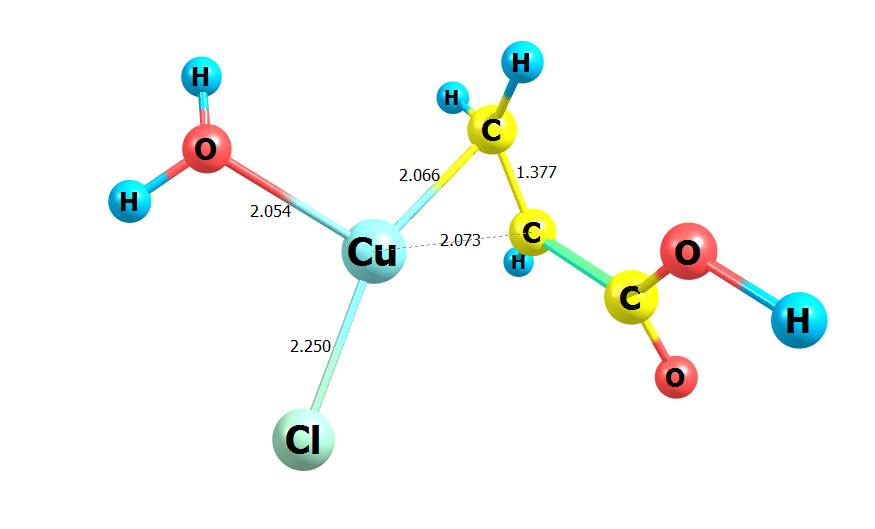
The laws of the combined action of σ- and π-ligands on the electronic structure and thermodynamic parameters of Cu+ acidoaquachlorocomplexes were investigated using the method of quantum chemical modeling. It was found that anhydrous chloride complexes with molecules of unsaturated organic acids (acrylic, maleic, fumaric) have the best energy characteristics. They achieve the maximum binding energies of the central atom with the chloride ion (151 ± 2 kJ/mol) and the organic ligand (130 ± 1 kJ/mol), which are practically independent of the nature of the acid. The addition of water molecules to [Cu+(L)(Cl−)] is energetically beneficial in all cases. The value of ΔEr depends on the nature of the organic acid, its form of existence (molecules, anions), and the number of water molecules. Therefore, it varies in a wide range of values (10–60 kJ/mol). Hydration promotes the transition from σ-bonding by the central atom of anionic forms of organic ligands to π-bonding. Stable π-complexes [Cu+(L)(Cl−)(H2O)] exist with all forms of the studied acids. At the same time, the transition from the molecular form of organic acids to the anionic one totally worsens both the energetics of σ-bonds of Сu+ with chlorine anions and water molecules, as well as the energetics of π-bonds. The antagonism of the combined action of σ-ligands in [Cu+(L)(Cl−)(H2O)] was quantified by the change in the effective charge of the central atom. It was shown that in complexes with the molecular form of the studied unsaturated acids, chlorine anions reduce the electron donation of water molecules by 86 %, and water molecules reduce the electron donation of Cl− by 35 %.
References
Obaleye J. A., Ajibola A. A., Bernardus V. B., Hosten E. C. & Ozarowski, A. (2020). Synthesis, spectroscopic, structural and antimicrobial studies of a dimeric complex of copper(II) with trichloroacetic acid and metronidazole. Inorganica Chimica Acta, 503(1), 119404. https://doi.org/10.1016/j.ica.2019.119404
Santiago P.H.O., Santiago M. B., Martins C. H. G., & Gatto, C. C. (2020). Copper(II) and zinc(II) complexes with Hydrazone: Synthesis, crystal structure, Hirshfeld surface and antibacterial activity. Inorganica Chimica Acta, 508(1), 119632. https://doi.org/10.1016/j.ica.2020.119632
Abbas, S. Y., Basyouni, W. M., & El‐Bayouki, K. A. (2018). Synthesis, characterization and antimicrobial activity of 5‐(arylazo) salicylaldimines and their copper (II) complexes. Applied Organometallic Chemistry, 32(2), 1–10. https://doi.org/10.1002/aoc.4032
Didenko, N. O., Ransky, A.P. (2018). Growth regulatory activity of copper (II) complexes with some thioamides. Bulletin of Vinnytsia Polytechnic Institute, 4, 28–35.
Świderski G., Wojtulewski S., Kalinowska M., Świsłocka R., Wilczewska A. Z., Pietryczuk A., ... & Lewandowskia, W. (2020). The influence of selected transition metal ions on the structure, thermal and microbiological properties of pyrazine-2-carboxylic acid. Polyhedron, 175(1), 114173. https://doi.org/10.1016/j.poly.2019.114173
Sousa I., Claro V., Pereira J. L., Amaral A.L., Cunha-Silva L., Castro B., Castro, ... & Gameiro, P. (2012). Synthesis, characterization and antibacterial studies of a copper(II) levofloxacin ternary complex. Journal of Inorganic Biochemistry, 110(1), 64-71. https://doi.org/10.1016/j.jinorgbio.2012.02.003
Łodyga-Chruscińska E., Pilo M., Zucca A., Garribba E., Klewicka E., Rowińska-Żyrek M., ... & Cheshchevik, V. T. (2018). Physicochemical, antioxidant, DNA cleaving properties and antimicrobial activity of fisetin-copper chelates. Journal of Inorganic Biochemistry, 180 (1), 101-118. https://doi.org/10.1016/j.jinorgbio.2017.12.006
Guz-Regnera K., Komarnicka U. K., Futoma-Kołoch B., Wernecki M., Cal M., Kozieł S., ... & Bugla-Płoskońska, G. (2020). Antibacterial activity and action mode of Cu(I) and Cu(II) complexes with phosphines derived from fluoroquinolone against clinical and multidrug-resistant bacterial strains. Journal of Inorganic Biochemistry, 210(1), 111124. https://doi.org/10.1016/j.jinorgbio.2020.111124
Vargalyuk, V. F., Polonskyy, V. A., Stets, O. S., Stets, N. V., Shchukin, A. I. (2014). Microbiological properties of copper-based dispersion obtained by cathode precipitation in the presence of acrylic acid. Bulletin of Dnipropetrovsk University. Series: Chemistry, 22(2), 47–51. https://doi.org/10.15421/081420
Skorik N. A., Filippova M. M., Bukholtseva E. I., & Malkov, V. S. (2015). Complex connections of cobalt (ІІ), copper (ІІ) and zinc with 2-methoxycarbonylaminohinasolone-4. Russian Journal of Inorganic Chemistry, 60(6), 729-735. https://doi.org/10.1134/S0036023615060157
Kondratenko, Y., Zolotarev, A.A., Ignatyev, Ugolrov V., & Kochina, T. (2020). Synthesis, crystal structure and properties of copper(II) complexes with triethanolamine and carboxylic acids (succinic, salicylic, cinnamic). Transition Metal Chemistry, 45(1), 71-81. https://doi.org/10.1007/s11243-019-00359-7
Oybek I. K., Ruzmetov A., Ibragimov A. B., Ashurov, J. M., Khasanov S. B. , Eshchanov E. U., & Ibragimov, B. T. (2022). Synthesis, crystal structure and Hirshfeld surface analysis of the binuclear Cu(II) complex with 4-nitrobenzoic acid and triethanolamine. Chemical Data Collections, 37, 100802B. https://doi.org/10.1016/j.cdc.2021.100802
Skorik N. A., Filippova M. M., Bukhol’tseva E. I., Mal’kov V. S. & Kurzina, I. A. (2015). Cobalt(II) and copper(II) complexes with carboxylic acids, imidazole, and 2-methylimidazole. Russian Journal of Inorganic Chemistry, 60(1), 729–735. https://doi.org/10.1107/S205698902200531X
Yanchak, А. I., Slyvka, Y. I., Kinzhybalo, V. V., Bednarchuk, T. J., & Myskiv, M. G. (2019). The First Copper(I) Halide π-Complexes with Allyl Derivatives of Urea and Parabanic Acid. Voprosy khimii i khimicheskoi tekhnologii, 3, 67–73. https:// doi.org/10.32434/0321-4095-2019-124-3-67-73
Slyvka, Yu. (2019). Copper(I) chloride and copper(I) perchlorate π-complexes with 2-allylthio-5-methyl-1,3,4-thiadiazole: synthesis and crystal structure. Visnyk of the Lviv University. Series Chemistry, 60(1), 155–162. https://doi.org/10.30970/vch.6001.155
Brathwaite A. D., Ward T. B., Walters R. S., & Duncan, M. A (2015). Cation−π and CH−π Interactions in the Coordination and Solvation of Cu+ (acetylene)nComplexes. J. Phys. Chem, 119 (22), 5658–5667. https://doi.org/10.1021/acs.jpca.5b03360
Slyvka, Y. I., Ardan, B. R., & Mys’kiv, M. G. (2018). Copper (I) chloride π-complexes with 2, 5-bis (allylthio)-1, 3, 4-thiadiazole: synthesis and structural features. Journal of Structural Chemistry, 59(2), 388–394. https://doi.org/10.1134/S0022476618020191
Ardan, B., Kinzhybalo, V., Slyvka, Y., Shyyka, O., Lukyanov, M., Lis, T., Myskiv, M. (2017). Ligand-forceddimerization of copper (I)–olefin complexes bearing a1, 3, 4-thiadiazole core. Acta Crystallographica Section C: Structural Chemistry, 73(1), 36–46. https://doi.org/10.1107/S2053229616018751
Vargalyuk, V. F., Polonskyy, V. A., Kurasova, Y. D. (2022). Quantum chemical modeling of Cu2+ acidochlorocomplexes containing anions of organic acids. Journal of Chemistry and Technologies, 30(1), 44-51.https://doi.org/10.15421/jchemtech.v30i1.253575
Vargalyuk, V. F., Polonskyy, V. A., Osokin, Y. S., (2020).Influence of maleic acid on the composition and structure of organocopper dispersions obtained bychemical and electrochemical reduction of Cu2+-ions. Journal of Chemistry and Technologies, 28(3), 231–241. https://doi.org/10.15421/082025
Cortés-Guzmán, F., & Bader, R. F. (2005). Complementarity of QTAIM and MO theory in the study of bonding in donor–acceptor complexes. Coordination Chemistry Reviews, 249(5–6), 633–662. https://doi.org/10.1016/j.ccr.2004.08.022
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., ... Cioslowski, D. J. (2010). Fox Gaussian 09, Revision C. 01. Gaussian Inc.
Biegler-König, F. B., Schönbohm, J., Bayles, D. (2001). AIM2000-a program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Lee, C., Yang, W., Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical review B, 37(2), 785–789. https://doi.org/10.1103/PhysRevB.37.785
Wachters, A. J. (1970). Gaussian basis set for molecular wavefunctions containing third‐row atoms. The Journal of Chemical Physics, 52(3), 1033–1036. https://doi.org/10.1063/1.1673095
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A., & Glushkov, V. N. (2019). Features of (dπ-pπ)-binding ofCu(I) ions with acrylic, maleic and fumaric acids inaqueous solution. Journal of Chemistry and Technologies, 27(2), 148–157. https://doi.org/10.15421/081916
Krishnan, R. B., Binkley, J. S., Seeger, R., & Pople, J. A. (1980). Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions.The Journal of Chemical Physics, 72(1), 650–654. https://doi.org/10.1063/1.438955
Frisch, M. J., Pople, J. A., Binkley, J. S. (1984). Self‐consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. The Journal of chemical physics,80(7), 3265–3269. https://doi.org/10.1063/1.447079
Barone, V., Cossi, M., Tomasi, J. (1998). Geometry optimization of molecular structures in solution by the polarizable continuum model. Journal of Computational Chemistry, 19(4), 404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Tomasi, J., Mennucci, B., Cammi, R. (2005). Quantum mechanical continuum solvation models. Chemicalreviews, 105(8), 2999–3094. https://doi.org/10.1021/cr9904009
Espinosa, E., Molins, E., Lecomte, C. (1998). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters, 285(3/4), 170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Mirzaeva, I. V., Kozlova, S. G., & Krisyuk, V. V. (2021). Quantum Chemical Study of the stability of Copper-Palladium complexes in the gas phase. Journal of Structural Chemistry, 62(1), 9–18. https://doi.org/10.1134/S0022476621010029
Faraji, S., Wang, B., Valencia, H. O., Frapper, G. (2021). Computational discovery of two-dimensional copper chalcogenides CuX (X = S, Se, Te). Physical Review Materials, 5(12), 124007. https://doi.org/10.1103/PhysRevMaterials.5.124007
Vargalyuk, V. F., Borschevich, A. O., Borschevich, L. V., Serediuk, V. A. (2017). Quantum-chemical analysis of formation reactions of Со2+ complexes. Bulletin of Dnipropetrovsk University. Series Chemistry, 25(1), 15–20. https://doi.org/10.15421/081703
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

