STUDY OF THE INFLUENCE OF DERIVATIVES 3-((6-R-QUINOLIN-4-YL)THIO)PROPANOIC ACID ON RHIZOGENESIS OF PINK ROSE (ROSA DAMASCENA MILL.) CLONES
DOI:
https://doi.org/10.15421/jchemtech.v30i4.265167Keywords:
Key words:. derivatives of quinoline and propanoic acid, stimulators of rhizogenesis, bioavailability factors, lipophilicity, toxicity, progressive mobility.Abstract
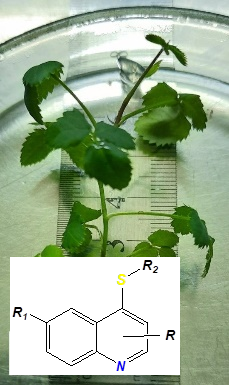
Today, in microclonal propagation of plants, there is a demand for effective and low-toxic rhizogenesis stimulators of plant explants. The use of such compounds significantly increases the efficiency of microclonal reproduction. The modern direction in the design of effective non-toxic substances is molecular modeling based on known natural and synthetic compounds. An important place as synthons for development is occupied by nitrogen-containing hetero-cycles, in particular quinoline. Results and discussion. Among the derivatives of 3-((6-R-quinolin-4-yl)thio) propanoic acid, the most toxic compounds were those that did not have alkoxy substituents in the 6th position of the quinoline cycle and no methyl radical in the 2nd position. Sodium salts are more toxic than the corresponding acids. This is due to the increase in water solubility of ionized compounds. Derivatives of 3-((6-R-quinolin-4-yl)thio)propanoic acid (sodium salt of 3-((quinolin-4-yl)thio)propanoic acid (QРА-5) showed the greatest toxic effect on the model of the study of progressive sperm motility ) and 3-((quinolin-4-yl)thio)propanoic acid (QРА-1), which would reduce this indicator by 25–30 % compared to intact. The toxicity assessment of the investigated com-pounds made it possible to determine a number of factors and factors of the structure of molecules, which affected the level of toxic action of 3-((6-R-quinolin-4-yl)thio) propanoic acid derivatives and directions for creating non-toxic growth stimulants in this series of 4-thioderivatives of quinoline. Conclusions. The investigated compounds showed a high stimulating effect on rhizogenesis in vitro in explants of pink rose (Rosa damascena Mill.) variety Lada. The selection of leader compounds for further testing of potential stimulators of rhizogenesis for microclonal propagation of ornamental plants was carried out. The obtained results are of high practical importance for obtaining and further introduction of new effective, low-toxic, less expensive substances for plant reproduction, in the conditions of microclonal production.
References
Anjos, J. D., Stefanello, C. A., Vieira, L. D., Polesi, L. G., Guerra, M. P., Fraga, H. P. D. (2021). The cytokinin 6-Benzylaminopurine improves the formation and development of Dryadella zebrina (Orchidaceae) in vitro shoots. Brazilian Journal of Botany, 44(4), 811–819. https://doi.org/10.1007/s40415-021-00753-5
Aremu, A. O., Dolezal, K., Van Staden, J. (2015). New cytokinin-like compounds as a tool to improve rooting and establishment of micropropagated plantlets. Acta Horticulturae, 6th International Symposium on Production and Establishment of Micropropagated Plants, Sanremo, Italy, 497-503. doi 10.17660/ActaHortic.2017.1155.73
Awada, R., Verdier, D., Froger, S., Brulard, E., Maraschin, S. D., Etienne, H., Breton, D. (2020). An innovative automated active compound screening system allows high-throughput optimization of somatic embryogenesis in Coffea arabica. Scientific Reports, 10(1). 810. https://doi.org/10.1038/s41598-020-57800-6
Bergmann, B. A., Whetten, R. (1998). In vitro rooting and early greenhouse growth of micropropagated Paulownia elongata shoots. New Forests, 15, 127–138.
Bogdan, A. M., Brazhko, O. A., Labenska, I. B. (2019). Acute toxicity and hypoglycemic activity of 7-chloro-4-thio-substituted quinoline. Bulletin of Zaporizhzhia National University. Biological Sciences, 1, 23–30, (in Ukrainian). https://doi.org/10.26661/2410-0943-2019-1-03
Brazhko, O. A., Omelyanchik, L. O., Zavgorodniy, M. P., Martynovsky, M. P. (2013). Chemistry and biological activity 2(4)-thioquinolines and 9-thioacridines. Zaporizhzhya: Zaporizhzhia National University. (in Ukrainian).
Brazhko, O. A., Gencheva, V.I., Kornet, M.M., Zavgorodniy, M. P. (2020). Modern Aspects Of Drugs Creation Based On QuS-Program Development. LAMBERT Academic Publishing. Republic of Moldova.
Brazhko, O. A., Zavgorodniy, M. P., Kornet, M. M., Lagron, A. V., Dobrodub, I. V. (2018). Synthesis and biological activity of derivatives (2-methyl(phenyl)-6-r-quinolin-4-yl-sulphanyl)carboxylic acid. Science Review. 7(7), 8–10.
Brazhko, O. A., Kornet, M. M., Zavgorodniy, M. P. Pat. Ukraine. 97937 No. 2011 11474.
Chalageri, G., Dhananjaya, S. P., Raghavendra, P., Kumar, L. M. S., Babu, U. V., Varma, S. R. (2019). Substituting plant vegetative parts with callus cell extracts: сase study with Woodfordia fruticosa Kurz. – A potent ingredient in skin care formulations. South African Journal of Botany, 123, 351-360. https://doi.org/10.1016/j.sajb.2019.03.002
Derevianko, N. P, Brazhko, O. A, Zavgorodniy, M. P, Vasilieva, T. M. (2016). Efficiency and safety of new plant growth stimulants based on heterocarboxylic acid derivatives. Agroecological journal, 3, 100–104. (in Ukrainian). https://doi.org/10.33730/2077-4893.3.2016.248874
Dhooghe, E., Lootens, P., Van Poucke, C., De Keyser, E., Van Huylenbroeck, J. (2021). Antitranspirants putrescine and abscisic acid improve acclimatization of micropropagated spathiphyllum 'lima' regel. Propagation of Ornamental Plants, 21(3), 67–77.
Fokina, A. V., Satarova, T. M., Smetanin, V. T., & Kucenko, N. I. (2018). Optimization of microclonal propagation in vitro of oregano (Origanum vulgare). Biosystems Diversity, 26(2), 98–102. https://doi.org/10.15421/011815
Grishchenko, O. V., Subbotin, E. P., Gafitskaya, I. V., Vereshchagina, Y. V., Burkovskaya, E. V., Khrolenko, Y. A., Kulchin, Y. N. (2022). Growth of Micropropagated Solanum tuberosum L. Plantlets under Artificial Solar Spectrum and Different Mono- and Polychromatic LED Lights. Horticultural Plant Journal, 8(2), 205–214. https://doi.org/10.1016/j.hpj.2021.04.007
Ilczuk, A., Jagiello-Kubiec, K., Jacygrad, E. (2013). The effect of carbon source in culture medium on micropropagation of common ninebark (Physocarpus opulifolius (L.) Maxim.) 'Diable D'or'. Acta Scientiarum Polonorum-Hortorum Cultus, 12(3), 23–33.
Ivashchuk, O. A., Batlutskaya, I. V., Maslova, E. V., Shcherbinina, N. V., Shamraev, A. A., Gaidai, P. A. (2018). Approaches to conservation of biodiversity of rare and endangered medicinal plants on the basis of microclonal multiplication with optimization of parameters by methods of neural network modeling. Research Journal of Pharmaceutical Biological and Chemical Sciences, 9(5), 2347–2356.
Kalinin F. L. (1984). Biologically active substances in crop production. Kyiv: Scientific opinion. 316 p.
Kornet, M. M., Brazhko, О. А., Zavhorodniy, M. P., Tkach, V. V., Kruglyak, O. S., de Oliveira, S. C. (2021). Electrochemical determination of antioxidant activity of new 4-thiosubstituted quinoline derivatives with potential radioprotecting properties. Biointerface Research in Applied Chemistry. 11(2), 9148-9156. https://doi.org/10.33263/BRIAC112.91489156
Kornet, M. M., Dudareva, G. F., Gencheva, V. I., Klimova, O. O., Peretiatko, V. V., Brazhko, O. A. (2020). Growth-regulatory activity of 2-methyl-4-thioquinoline derivatives. Ukrainian Journal of Ecology. 10(2), 279-283. doi: 10.15421/2020_97.
Kornet, M. M. (2012). Biological activity of S-(quinolin-4-yl)-L-cysteine derivatives and their structural analogues. The dissertation of Cand. biol. Science. (In Ukrainian).
Kulak, V., Longboat, S., Brunet, N. D., Shukla, M., & Saxena, P. (2022). In vitro technology in plant conservation: relevance to biocultural diversity. Plants-Basel, 11(4), Article 503. https://doi.org/10.3390/plants11040503
Maistrenko, G. G., Krasnoborov, I. M. (2009). Microclonal propagation and biological features of Scrophularia umbrosa Dumort. Cultured in vitro. Contemporary Problems of Ecology, 2(6), 501–505. https://doi.org/10.1134/s1995425509060010
Martin, T. M. (2016). Toxicity Estimation Software Tool (TEST). Washington: U.S. Environmental Protection Agency, https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test.
Matskevich, V. V, Podgaetsky, A. A, Filipova, L. M. (2019). Microclonal propagation of certain plant species (technology protocols). Scientific and practical manual. Bila Tserkva: BNAU. https://doi.org/10.14258/turczaninowia.23.3.4
Metelytsia, L., Hodyna, D., Dobrodub, I., Semenyuta, I., Zavhorodnii, M., Blagodatny, V., Brazhko, O. (2020). Design of (quinolin-4-ylthio)carboxylic acids as new Escherichia coli DNA gyrase B inhibitors: machine learning studies, molecular docking, synthesis and biological testing. Computational Biology and Chemistry , 85, 107224. doi: 10.1016/j.compbiolchem.2020.107224
Mikhovich, Z. E., Teteryuk, L. V. (2020). In vitro culture of the Ural endemic Gypsophila uralensis Less. (Caryophyllaceae). Turczaninowia, 23(3), 29–35. doi: 10.14258/turczaninowia.23.3.4
Misyri, V., Tsekouras, V., Iliopoulos, V., Mavrikou, S., Evergetis, E., Moschopoulou, G., Haroutounian, S. A. (2021). Farm or lab? Chamazulene content of Artemisia arborescens (Vill.) L. essential oil and callus volatile metabolites isolate. Industrial Crops and Products, 160, 113114. https://doi.org/10.1016/j.indcrop.2020.113114
Orlikowska, T., Zawadzka, M., Zenkteler, E., Sobiczewski, P. (2012). Influence of the biocides PPM (TM) and Vitrofural on bacteria isolated from contaminated plant tissue cultures and on plant microshoots grown on various media. Journal of Horticultural Science & Biotechnology, 87(3), 223–230. https://doi.org/10.1080/14620316.2012.11512856
Pereira, V. J., Asmar, S. A., Biase, N. G., Luz, J. M. Q., de Melo, B. (2018). Statistics applied to plant micropropagation: a critical review of inadequate use. Bioscience Journal, 34(5), 1308–1318. https://doi.org/10.14393/BJ-v34n5a2018-38778
Rodrigues, V., Kumar, A., Gokul, S., Verma, R. S., Rahman, L. U., & Sundaresan, V. (2020). Micropropagation, encapsulation, and conservation of Decalepis salicifolia, a vanillin isomer containing medicinal and aromatic plant. In Vitro Cellular & Developmental Biology-Plant, 56(4), 526–537. https://doi.org/10.1007/s11627-020-10066-z
Yakovleva-Nosar, S. O., Derevyanko, N. P., Yevlash, A. S., Brazhko, O. A., Zavhorodnii, M. P., Tkach, V. V., Yagodynets, P. I. (2022). A search of the efficient s-hetarylsuccinate landscape design plant growth stimulators. Biointerface Research in Applied Chemistry, 12(1), 465–469. doi: 10.33263/BRIAC121.465469
Stefanov, O. V., (editor 2001). Preclinical study of drugs (methodical recommendation). Kyiv, Avitsena. (in Ukrainian).
Tiuzikov, I. A. (2013). Metabolic syndrome and male infertility (literature review). Andrology and Genital Surgery, 2, 5–10. (in Russian). doi: 10.17650/2070-9781-2013-2-7-10
Tung, H. T., Bao, H. G., Cuong, D. M., Ngan, H. T. M., Hien, V. T., Luan, V. Q., Nhut, D. T. (2021). Silver nanoparticles as the sterilant in large-scale micropropagation of chrysanthemum. In Vitro Cellular & Developmental Biology-Plant, 57(6), 897–906. https://doi.org/10.1007/s11627-021-10163-7
Yegizbayeva, T. K., Yausheva, T. V., Oleichenko, S. N., & Licea-Moreno, R. J. (2020). Influence of nutrition compositions on microclonal propagation different genotypes of the Walnut Juglans Regia l. Bulletin of the National Academy of Sciences of the Republic of Kazakhstan (1), 105–112. https://doi.org/10.32014/2020.2518-1467.13
Zakharova, O., Kolesnikova, E., Kolesnikov, E., Yevtushenko, N., Morkovin, V., Gusev, A. (2020, Oct 23). CuO nanoparticles effects on poplar(x)aspen hybrid clones at various stages of microclonal propagation. International Forestry Forum on Forest Ecosystems as Global Resource of the Biosphere - Calls, Threats, Solutions (Forestry), 595(1), 012001. doi: 10.1088/1755-1315/595/1/012001
Ziauka, J., Kuusiene, S., & Silininkas, M. (2013). Fast growing aspens in the development of a plant micropropagation system based on plant-produced ethylene action. Biomass & Bioenergy, 53, 20–28. https://doi.org/10.1016/j.biombioe.2013.01.005
Abu-Hashem, A. A., Abdelgawad, A. A. M., Hussein, H. A. R., & Gouda, M. A. (2022). Synthetic and Reactions Routes to Tetrahydrothieno 3,2-b Quinoline Derivatives (Part IV). Mini-Reviews in Organic Chemistry, 19(1), 74–91. https://doi.org/10.2174/1570193x18666210218212719
Hassan, M. M., & Alzandi, A. R. A. (2020). Synthesis, structure elucidation and plants growth promoting effects of novel quinolinyl chalcones. Arabian Journal of Chemistry, 13(7), 6184–6190. https://doi.org/10.1016/j.arabjc.2020.05.024
Koprulu, T. K., Okten, S., Atalay, V. E., Tekin, S., & Cakmak, O. (2021). Biological activity and molecular docking studies of some new quinolines as potent anticancer agents. Medical Oncology, 38(7), 84. https://doi.org/10.1007/s12032-021-01530-w
Lenin, S., Sujatha, R., & Shanmugasundaram, P. (2022). Pharmacological Properties And Bioavailability Studies Of 3-Methyl Quinoline. International Journal of Life Science and Pharma Research, 12(1), L100–L104. https://doi.org/10.22376/ijpbs/lpr.2022.12.1.L100-104
Lu, W., Chen, J. C., Shi, J. Z., Xu, L., Yang, S. L., & Gao, B. H. (2021). A novel quinoline-based turn-on fluorescent probe for the highly selective detection of Al (III) and its bioimaging in living cells, plants tissues and zebrafish. Journal of Biological Inorganic Chemistry, 26(1), 57–66. https://doi.org/10.1007/s00775-020-01836-6
Namitha, R., Priyadarshini, G. S., & Selvi, G. (2021). Pharmacological Studies on Novel Triazino Quinolines. Advances in Pharmacology and Pharmacy, 9(4), 81–86. https://doi.org/10.13189/app.2021.090401
Salem, M. A., Abu-Hashem, A. A., Abdelgawad, A. A. M., Gouda, M. A. (2021). Synthesis and reactivity of thieno 2,3-b quinoline derivatives (Part II). Journal of Heterocyclic Chemistry, 58(9), 1705-1740. https://doi.org/10.1002/jhet.4269
Singh, K., Sharma, R., Sahare, H. (2022). Implications of synthetic chemicals and natural plant extracts in improving vase life of flowers. Scientia Horticulturae, 302, 111133. https://doi.org/10.1016/j.scienta.2022.111133
Vostrikova, T. V., Kalaev, V. N., Potapov, A. Y., Manakhelokhe, G. M., & Shikhaliev, K. S. (2021). Use of new compounds of the quinoline series as growth and yield stimulants of agricultural crop. Periodico Tche Quimica, 18(38), 123-136. https://doi.org/10.52571/PTQ.v18.n38.2021.9_VOSTRIKOVA_pgs_123_136.pdf
Yepes, A. F., Quintero-Saumeth, J., Cardona-Galeano, W. (2021). Biologically active quinoline-hydrazone conjugates as potential Trypanosoma cruzi DHFR-TS inhibitors: docking, molecular dynamics, MM/PBSA and drug-likeness studies. Chemistryselect, 6(12), 2928–2938. https://doi.org/10.1002/slct.202100238
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

