ETHANOLAMINE AND PENTYL ACETATE INTERACTION CATALYZED BY CATION EXCHANGE RESIN: KINETIC INSIGHT
DOI:
https://doi.org/10.15421/jchemtech.v31i1.267433Keywords:
ethanolamine; pentyl acetate; N-(2-hydroxyethyl)acetamide; 2-aminoethyl acetate; 2-(acetylamino)ethyl acetate; kinetic; aminolysis; transesterification; cation exchange resinAbstract
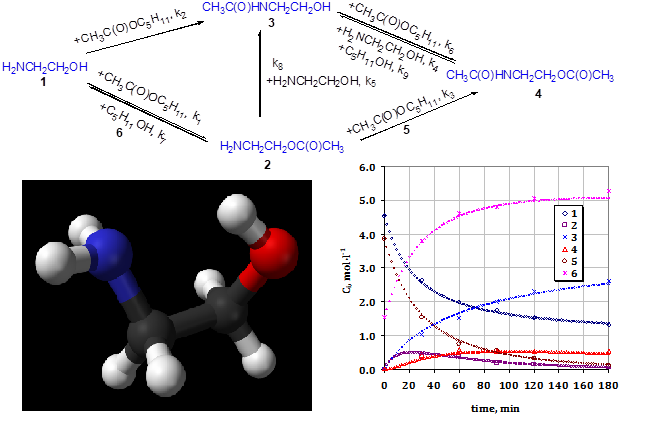
We report the scheme of ethanolamine, pentyl acetate, and their interaction products in transesterification, aminolysis, and O-N-acyl migration reactions catalyzed by H-cation exchange resin. The routes of N-(2-hydroxyethyl)acetamide, 2-aminoethyl acetate, and 2-(acetylamino)ethyl acetate formation indicate that N-(2-hydroxyethyl)acetamide is the final product. The determined rate constant values for the proposed quasi-homogeneous reaction model indicate the significant role of aminolysis reaction by ethanolamine and pentyl acetate interaction catalyzed by H-cation exchange resin. In particular, high values of the rate constant of aminolysis reactions indicate a high rate of amide formation during the interaction of ethanolamine and pentyl acetate, 2-aminoethyl acetate, or 2-(acetylamino)ethyl acetate. The proposed kinetic model adequately describes the N-(2-hydroxyethyl)acetamide obtaining process from ethanolamine and pentyl acetate. The values of the considered reactions' pre-exponential factors, rate constants, energy activation, entropy and enthalpy activation were calculated. In particular, the reaction of N-(2-hydroxyethyl)acetamide formation by aminolysis of pentyl acetate with ethanolamine has the lowest activation energy (15.8 kJ∙mol–1), and the reaction of 2-(acetylamino)ethyl acetate formation by N-(2-hydroxyethyl)acetamide with pentyl acetate transesterification has the highest activation energy (89.1 kJ∙mol–1). A linear relationship was revealed between the pre-exponential factor logarithms of reaction rate constants and activation energies and between the enthalpy and entropy activation. We assume the compensatory effect and the absence of an isokinetic relationship for the entire set of reactions. The study results are the basis for modeling the N-(2-hydroxyethyl)acetamide obtaining process from ethanolamine and pentyl acetate.
References
Mishra, S., Tyagi, V.K. (2007). Ester Quats: The Novel Class of Cationic Fabric Softeners. J. Oleo Sci., 56(6), 269–276. https://doi.org/10.5650/jos.56.269
Petrosino, S., Iuvone, T., Di Marzo, V. (2010). N-palmitoyl-ethanolamine: Biochemistry and new therapeutic opportunities, Biochimie, 92, 724–727. https://doi.org/10.1016/j.biochi.2010.01.006
Hansen, H. S., Diep, T. A. (2009). N-acylethanolamines, anandamide and food intake, Biochem. Pharmacol., 78, 553–560. https://doi.org/10.1016/j.bcp.2009.04.024
Topilnytskyy, P., Romanchuk, V., Yarmola, T. (2018). Production of corrosion inhibitors for oil refining equipment using natural components, Chem. Chem. Technol., 2(3), 400–404. https://doi.org/10.23939/chcht12.03.400].
Dinesh, K., Amjad, A. (2015). Direct synthesis of fatty acid alkanolamides and fatty acid alkyl esters from high free fatty acid containing triglycerides as lubricity improvers using heterogeneous catalyst, Fuel, 159, 845–853. https://doi.org/10.1016/j.fuel.2015.07.046
Melnyk, S., Danyliuk, R., Melnyk, Yu., Reutskyy V. (2018). The reaction of oleic acid with a mixture of ethanolamines, Chem. Chem. Technol., 12(1), 13–17. https://doi.org/10.23939/chcht12.01.013
Wang, X., Wang, T., Wang, X. (2012). An Improved Method for Synthesis of N-stearoyl and N-palmitoylethanolamine, J. Am. Oil. Chem. Soc., 89, 1305–1313. https://doi.org/10.1007/s11746-012-2017-y.
Wang, X., Chen, Y., Jin, Q., Huang, J., Wang, X. (2013). Synthesis of Linoleoyl Ethanolamide. J. Oleo Sci., 62(6), 427–433. https://doi.org/10.5650/jos.62.427
Mahadevan, S., Venkatasubban, K. (2012). WO Patent No 2012/148624 Al. World Intellectual Property Organization, International Bureau.
Markey, S. P., Dudding, T., Wang, T.-C. L. (2000). Base- and acid-catalyzed interconversions of O-acyl- and N-acyl-ethanolamines: a cautionary note for lipid analyses, J. Lipid Res., 41(4), 657–662. https://doi.org/10.1016/S0022-2275(20)32414-7
Berčíková, M., Lád, J., Hrádková, I., Kumherová, M., Šmidrkal, J. (2021). Reaction of Fatty Acid Methyl Ester with Monoethanolamine and Diethanolamine, Tenside Surfactants Detergents, 58(4), 287–292. https://doi.org/10.1515/tsd-2020-2328
Thabuis, C., Tissot-Favre, D., Bezelgues, J.-B., Martin, J.-C., Cruz-Hernandez, C., Dionisi, F., Destaillats F. (2008). Analysis of chemically synthesized oleoylethanolamide by gas-liquid chromatography. J. Chromatogr. A, 1202, 216–219. https://doi.org/10.1016/j.chroma.2008.07.008
Peng, X. (2017). PRC Patent Application No CN 106631859 A. State Intellectual Property Office of the P.R.C.
Ohshima, Y., Imoto, H., Fujiu A. (1997). German Patent No DE 19648513 A1. Deutshe Patentamt.
Caldwell, N., Jamieson, C., Simpson, I., Tuttle T. (2013). 0rganobase-Catalyzed Amidation of Esters with Amino Alcohols, Org. Lett., 15(10), 2506–2509. https://doi.org/10.1021/ol400987p
Movassaghi, M., Schmid M. A. (2005). N-Heterocyclic carbene-catalyzed amidation of unactivated esters with amino alcohols, Org. Lett., 7(12), 2453–2456. https://doi.org/10.1021/ol050773y
Lei, X., Lu, W., Peng, Q., Li, H., Chen, T., Xu, S., Zhang, F. (2011). Activated MgAl-layered double hydroxide as solid base catalysts for the conversion of fatty acid methyl esters to monoethanolamides, Appl. Cat. A: General, 399, 87–92. https://doi.org/10.1016/j.apcata.2011.03.042
Chintareddy, V. R., Ho, H.-A., Sadow, A. D., & Verkade, J. G. (2011). Polymer-mounted N3=P(MeNCH2CH2)3N: a green, efficient and recyclable catalyst for room-temperature transesterifications and amidations of unactivated esters, Tetrahedron Lett., 52(49), 6523–6529. https://doi.org/10.1016/j.tetlet.2011.09.102
Melnyk, Yu., Melnyk, S., Palyukh, Z., Dzinyak, B. (2018). Research into transesterification of triglycerides by aliphatic alcohols C2–C4 in the presence of ionites. East.-Eur. J. Enterp. Technol., 1/6(94), 10–16. https://doi.org/10.15587/1729-4061.2018.122938
Trus, I, Gomelya, M., Tverdokhlib, M. (2021). Evaluation of the contribution of ion exchange in the process of demanganization with modified cation exchange resin KU-2-8, Journal of Chemistry and Technologies, 29(4), 540–548. https://doi.org/10.15421/jchemtech.v29i4.242561
Melnyk, S., Danyliuk, R., Melnyk, Yu., Stadnytska, N. (2022). Study of the Pentyl Acetate and Ethanolamine Catalytic and Non-Catalytic Interaction. Journal of Chemical Technology and Metallurgy, 57(3), 439–450.
Melnyk, S., Dzinyak, B. (2015). Selectivity of formation and yield of dicarboxylic acid mono- and diesters under stationary conditions. Chem. Chem. Technol., 9(3), 325–332. https://doi.org/10.23939/chcht09.03.325
Schmid R., Sapunov V. N. (1982). [Non-formal Kinetics: in Search for Chemical Reaction Pathways]. Weinheim: Verlag Chemie.
Dudding, T., Wang, T. C. (2000). Base- and acid-catalyzed interconversions of O-acyl- and N-acyl-ethanolamines: a cautionary note for lipid analyses, J. Lipid Res., 41(4), 657–662. https://doi.org/10.1016/S0022-2275(20)32414-7
Glavan, D., Gremasco, Y., Gomes Mantovani, A. C., Bona, E., Killner, M., Borsato, D. (2020). Kinetic study of the transesterification reaction by artificial neural networks and parametric particle swarm optimization, Fuel, 267, 1172218. https://doi.org/10.1016/j.fuel.2020.117221
Melnyk, S. R., Khlibkevych, U. I., Melnyk, Y. R., Mahorivska, H. Ya. (2021). Kinetic research and modeling of benzoic acid esterification process, Journal of Chemistry and Technologies, 29(4), 559–569. https://doi.org/10.15421/jchemtech.v29i4.241445
Exner, O. (1997). How to get wrong results from good experimental data: a survey of incorrect application of regression, J. Phys. Org. Chem, 10, 797–813. https://doi.org/10.1002/(SICI)1099-1395(199711)10:11<797::AID-PCA951>3.0.CO;2-K
Cornish-Bowden A. (2017). Enthalpy–entropy compensation and the isokinetic temperature in enzyme catalysis, Biosci. 42(4), 665. https://doi.org/10.1007/s12038-017-9719-0
Liu L., Guo Q.-X. (2001). Isokinetic Relationship, Isoequilibrium Relationship, and Enthalpy−Entropy Compensation. Chem. Rev., 101(3), 673–695. https://doi.org/10.1021/cr990416z
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

