SEARCH OF REGULATORS AMONG (7-CHLOROQUINOLINE-4-YLTHIO)CARBONIC ACIDS AND TRIAZOLES OF ANTHRACENDIONE FOR MICROCLONAL PROPAGATION OF PLANTS
DOI:
https://doi.org/10.15421/jchemtech.v31i1.271400Keywords:
мікроклональне розмноження; триазоли; хінолінкарбонові кислоти; токсичність; рострегуляториAbstract
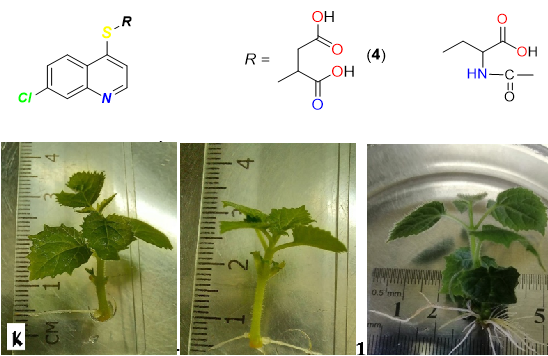
The use of rhizogenesis stimulators during microclonal propagation of plants significantly increases efficiency and reduces costs. The modern direction in the design of effective non-toxic substances is molecular modeling based on known natural and synthetic compounds. An important place as synthons for development is occupied by nitrogen-containing heterocycles, in particular, pyridine, quinoline, anthracenedione. The growth-stimulating properties of (7-chloroquinoline-4-ylthio)carboxylic acids and new triazole derivatives based on anthracenedione were investigated. Lipophilicity was analyzed using the ACD-I-Labs computer program. The log P for neutral forms and the value of the distribution coefficient log D at pH = 7 for the investigated compounds were determined. Determination of the precise action of compounds in silico was carried out with the help of computer programs GUSAR (Germany), TEST (USA) and on the in vitro model of the study of progressive sperm motility. The effect on rhizogenesis was evaluated by the method of microclonal propagation of plants in the conditions of an in vitro microclonal laboratory of pink rose (Rosa damascena Mill.) explants of the Lada variety.
References
Zavhorodnii, M.; Derevianko, N.; Shkopynska, T.; Kornet, M.; Brazhko O. (2022). Influence of derivatives of 2-((6-r-quinolin-4-yl)thio)acetic acid on rhizogenesis of Paulownia clones. Regulatory Mechanisms in Biosystems, 13(3), 213-218. https://doi: 10.15421/022227
Malik, E.; Muller, C. (2016). Anthraquinones As Pharmacological Tools and Drugs. Medicinal research reviews. 36(4), 705-748. https://doi.org/10.1002/med.21391
Abu-Hashem, A. A., Abdelgawad, A. A. M., Hussein, H. A. R., & Gouda, M. A. (2022). Synthetic and Reactions Routes to Tetrahydrothieno 3,2-b Quinoline Derivatives (Part IV). Mini-Reviews in Organic Chemistry, 19(1), 74-91. https://doi.org/10.2174/1570193x18666210218212719
Hassan, M. M., & Alzandi, A. R. A. (2020). Synthesis, structure elucidation and plants growth promoting effects of novel quinolinyl chalcones. Arabian Journal of Chemistry, 13(7), 6184-6190. https://doi.org/10.1016/j.arabjc.2020.05.024
Metelytsia, L., Hodyna, D., Dobrodub, I., Semenyuta, I., Zavhorodnii, M., Blagodatny, V., Brazhko, O. (2020). Design of (quinolin-4-ylthio)carboxylic acids as new Escherichia coli DNA gyrase B inhibitors: machine learning studies, molecular docking, synthesis and biological testing. Computational Biology and Chemistry, 85, 107224. https://doi.org/10.1016/j.compbiolchem.2020.107224.
Kang, S.K., Woo, J., Cho, S., Lee, S,E., Kim, Y.K., Yoon, S.S. (2019). Synthesis of Benzo[g]quinoline Derivatives and Their Electroluminescent Properties. Journal of Nanoscience and Nanotechnology. 19(8), 4543-8 https://doi.org/10.1166/jnn.2019.16687.
Koprulu, T. K., Okten, S., Atalay, V. E., Tekin, S., & Cakmak, O. (2021). Biological activity and molecular docking studies of some new quinolines as potent anticancer agents. Medical Oncology, 38(7), Article 84. https://doi.org/10.1007/s12032-021-01530-w
Namitha, R., Priyadarshini, G. S., & Selvi, G. (2021). Pharmacological Studies on Novel Triazino Quinolines. Advances in Pharmacology and Pharmacy, 9(4), 81-86. https://doi.org/10.13189/app.2021.090401
Kornet, M.M., Brazhko, О.А., Zavhorodniy, M.P., Tkach, V.V., Kruglyak, O.S., de Oliveira, S.C. (2021). Electrochemical determination of antioxidant activity of new 4-thiosubstituted quinoline derivatives with potential radioprotecting properties. Biointerface Research in Applied Chemistry. 11(2), 9148-9156. https://doi.org/10.33263/BRIAC112.91489156
Shupeniuk, V., Taras, T., Sabadakh, O., Luchkevich, E., Matkivsky, M. (2021). Methods of synthesis of hydroxyanthraquinone derivatives and their biological activity. Journal of Chemistry and Technologies, 29(2). 219–231. doi: 10.15421/jchemtech.v29i2.225941
Shupeniuk, V., Nepolraj A., Taras, T., Sabadakh, O., Matkivsky, M., Luchkevich, E. (2022). Іn-silico study of anthraquinone derivatives as probable inhibitors of COVID-19. Journal of Chemistry and Technologies, 30(2). 151–158. doi: 10.15421/jchemtech.v30i2.244728
Shupeniuk, V., Taras, T., Sabadakh, O., Luchkevich, E., Matkivskyi, M., Kutsyk R. (2022). Synthesis and antimicrobial activity of nitrogen-containing anthraquinone derivatives. Iraqi Journal of Pharmaceutical Sciences 31(2), P. 193–201. DOI: https://doi.org/10.31351/vol31iss2pp193-201.
Brazhko, O.A., Zavgorodniy, M. P., Dobrodub, I.V., Omelyanchik, L.O., Gencheva, V.I., Novosad, N.V., Brazhko, O.O., Stalemate. on utility model 60110 Ukraine, IPC (2011.01) С 07 Д 215/18, 219/04, 221/00, №u201013975, Method of obtaining α- (heteryl- (thio)) - succinic acid; declared 23.11.2010; Publ. 10.06.2011 - Bull. № 11. – 10.
Brazhko, O.A., Zavgorodniy, M.P., Kornet, M.M., Lagron, A.V., Dobrodub, I.V., 2018 Synthesis and biological activity of derivatives (2-methyl(phenyl)-6-r-quinolin-4-yl-sulphanyl)carboxylic acid. Science Review. 7(7), 8-10.
Tiuzikov, I. A. (2013). Metabolic syndrome and male infertility (literature review). Andrology and Genital Surgery. 2, 5–10. DOI: 10.17650/2070-9781-2013-2-7-10
Stefanov, O.V., editor. 2001 Preclinical study of drugs (methodical recommendation). Kyiv, Avitsena. Ukrainian.
Murashige T., Scoog F. (1962). A revised medium for rapid, growth and bioassays with tobacco tissue cultures. Physiol. Plantarum, 15(3), 473.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

