PHASE EQUILIBRIA IN THE La2O3- Lu2O3- Er2O3 SYSTEM AT 1500 AND 1600 °С
DOI:
https://doi.org/10.15421/jchemtech.v31i1.271493Keywords:
фазові рівноваги, лантан, лютецій, ербій, параметри ґраткиAbstract
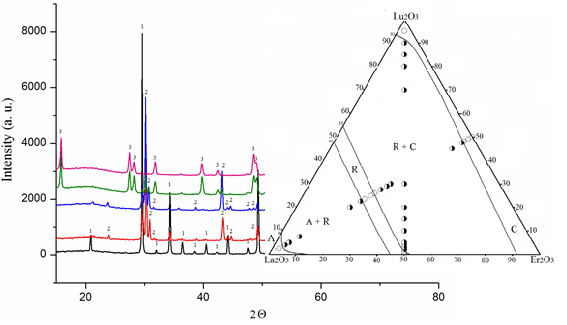
The phase relations in the La2O3–Lu2O3–Er2O3 ternary system at 1500 and 1600 °C were studied in the whole concentration range by X-ray diffraction (XRD) and scanning electron microscopy (SEM). Oxides of La, Lu, and Er (99.99 %) were used as starting substances. The samples were prepared with a concentration step of 1-5 mol. %. The oxides were dissolved in HNO3 (1:1) followed by evaporation of the solutions and decomposition of nitrates at 800 °C for 2 hours. The samples were heat treated at 1500 °C (for 70 h), and 1600 °C (for 10 h) in air. The phase composition of the test samples studied by X-ray diffraction (XRD, DRON-3), microstructural phase and electron microprobe X-ray (Superprobe-733, JEOL, Japan, Palo Alto, CA) analyses. Solid solutions based on various polymorphic forms of original oxides and ordered LaLuO3 (LaErO3) phases were detected in the system. No new phases were found in the system. The isothermal cross-sections of the La2O3–Lu2O3–Er2O3 phase diagram at 1500 and 1600 °C are characterized by the presence of three single-phase (A-La2O3, R, C-Lu2O3 (Er2O3)) and two two-phase (C+ R, A + R) regions. The system forms continuous series of solid solutions based on the cubic modification of C-Lu2O3(Er2O3) and the ordered perovskite-type phase (R-phase). Solubility limits are determined and concentration dependences of periods also lattice parameters of the unit cell of phases formed in the system are constructed. The range of homogeneity of solid solutions based on the R-phase extends from 46 to 54 mol % La2O3 at 1500 °C and from ~48 to 54 mol % La2O3 at 1600 °C. Lutetium and erbium oxides form an continuous series of C-REE oxide solid solutions.
References
Zatsepin, A., Kuznetsova, Y., Spallino, L., Pustovarov, V., Rychkov, V. (2016) Photosensitive defects in Gd2O3 – advanced material for solar energy conversion, Energy Procedia, 102, 144–151. https://doi.org/10.1016/j.egypro.2016.11.329.
Wang, S.F., Zhang, J., Luo, D. W., Gu, F., Tang, D. Y., Dong, Z. L., Que, W. X., Zhang, T. S., Li, S., Kong, L.B. (2013). Transparent ceramics: Processing, materialsand applications, Prog. Solid State Chem., 41, 20–54. https://doi.org/10.1016/j.progsolidstchem.2012.12.002
Sanghera, J., Bayya, S., Villalobos, G., Kim, W., Frantz, J., Shaw, B., Sadowski, B., Miklos, R., Baker, C., Hunt, M., Aggarwal, I., Kung, F. (2011). Transparent ceramics for high-energy laser systems, Opt. Mater., 33, 511–518. https://doi.org/10.1016/j.optmat.2010.10.038
Chen, B. S., Yiquan, W. (2013). New opportunities for transparent ceramics, Amer. Ceram. Soc. Bull., 2, 32–37.
Akiyama, J., Sato, Y., Taira, T. (2010). Laser ceramics with rare-earth-doped anisotropic materials, Optics Lett., 35, 3598–3600. https://doi.org/10.1364/OL.35.003598
Taira T. (2011). Domain-controlled laser ceramics toward Giant Micro-photonics, Opt. Mater. Express., 1, 1040–1050. https://doi.org/10.1364/OME.1.001040
Qiu-Hong Yang , Hong-Xu Zhou, Jun Xu, Liang-Bi Su. (2008). Synthesis and luminescence characterization of cerium doped Lu2O3-Y2O3-La2O3 solid solution transparent ceramics, Optics express., 16, 12295. doi:10.1364/oe.16.012290.
Chen, Y., Lin, X., Lin, Y., Luo, Z. (2004). Spectroscopic properties of Yb3+ ions in La2(WO4)3 crystal, Solid State Communications, 132, 533–538.
Gong, X., Xiong, F., Lin, Y. (2007). Crystal growth and spectral properties of Pr3+ : La2(WO4)3, Materials Research Bulletin, 42 413–419.
Lakshminarasimhan, N., Varadaraju, U. V. (2006). Luminescent host lattices, LaInO3 and LaGaO3—A reinvestigation of luminescence of d10 metal ions, Materials Research Bulletin, 41, 724–731.
Muller-Buschbaum, Hk., Graebner, P. H. (1971). Zur Kristallstruktur von LaErO3 und LaLuO3, Z. Anorg. Allg. Chem., 386, 158–162 (in German).
Xiong, K., Robertson, J. (2009). Electronic structure of oxygen vacancies in La2O3, Lu2O3 and LaLuO3, Microelectr. En., 86(7-9), 1672–1675. https://doi.org/10.1016/j.mee.2009.03.016
Chudinovych, O. V., Zhdanyuk, N.V. (2020). Interaction of lanthanum oxides and lutetium at a temperature of 1500–1600 ⁰C, Ukrainian Chem. J., 86(3), 19–25. (in Ukrainian). https://doi.org/10.33609/0041-6045.86.3.2020.19-25
Kornienko, O.A., Chudinovych, O.V., Bykov, A.I., Samelyuk, A.V., Andrievskaya, E.R. (2019). Phase equilibria in the La2O3–Er2O3 system in the temperature range1100–1500°C, Powder Metall. Met. Ceram., 58 (1–2), 89–98. https://doi.org/0.1007/s11106-019-00051-6.
Schneider, S.I., Roth, R.S. (1960). Phase equilibria in systems involving the rare-earth oxides. Part II: solid state reactions in trivalent rare-earth oxide systems, J. of Res. National Bureau Standards. A: Physics and Chem., 64 (4), 317–332.
Foex, M., Traverse, J.P. (1966). Étude du polymorphisme des sesquioxydes de terres rares àhaute température, Bulletin de Minéralogie., 89(2), 184–205.
Coutures, J., Rouanet, A., Verges, R., Foex, M. (1976). Etude a haute temperature des systems formes par le sesquioxyde de lanthane et les sesquioxydes de lanthanides. I: dia-grammes de phases (1400 oC < T < TLiquide), J. Solid State Chem., 17(1–2), 172–182.
Zinkevich, Matvei (2007). Thermodynamics of rare earth sesguioxides, Prog. Mater. Sci., 52, 597–647.
Zhang, Y. (2016). Thermodynamic Properties of Rare Earth Sesquioxides, McGill University, Montreal, QC, Canada), 151.
Lopato, L.M., Shevchenko, A.V., Kushchevskii, A.V., Tresvyatskii, S.G. (1974). Polymorphictransformations of rare earth oxides at high temperatures, Izv. AN SSSR. Neorg. Mater., 10(8), 1481–1487.
Traverse, J.P.(1971). Etude du Polymorphisme des ses-quioxydes de terres rares. These. Grenoble.
Andrievskaya, E. R. (2010). [Phase equilibria in thesystems of hafnium, zirconium and yttrium oxides ofrare earth elements], Naukova dumka, Kiev. (in Russian).
Shannon, R. D. (1976). Revised effective ionic radii systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr. A., 32, 751–754.
Zinkevich, M. (2007). Thermodynamic Database for Rare Earth Sesquioxides, https://materialsdata.nist.gov/handle/11256/965.
Pavlik, A., Ushakov, S.V., Navrotsky, A., Benmore, C. J., Weber, R.J.K. (2017). Structure and thermal expansion of Lu2O3 and Yb2O3 up to the melting points, J. Nucl. Mater., 495, 385–391.
Axelrud, L.G, Grin, Yu. N., Zavaliy, P. Yu. (1990). Software Package for Structural Analysis of Crystals, CSD, General description, Lviv.
Chudinovych, O. V., Bykov, О. І., Sameliuk, A.V. (2021). Phase relation studies in the La2O3–Lu2O3–Yb2O3 system at 1500 °С, Journal of Chemistry and Technologies, 29(4), 485–494.
Chudinovych, O. V., Bykov, О. І. (2020). Interaction of yttrium, lanthanum and erbium oxides at the temperature of 1500°C, Voprosy Khimii i Khimicheskoi Tekhnologii, 4, 194-200.
Chudinovych, O. V., Andrievskaya, О. R., Bogatyryova, J. D., Kovylyaev, V. V., Bykov, O. I. (2021). Phase equilibria in the La2O3-Y2O3-Nd2O3 system at 1500 °С, J. Eur. Ceram. Soc., 41, 6606–6616. https://doi.org/10.1016/j.jeurceramsoc.2021.06.017
Chudinovych, O. V., Bykov, О. І., Sameliuk, A.V. (2021). Interaction of lanthanum, lutetium, and ytterbium oxides at 1600 °C, Powder Metallurgy and Metal Ceramics., 60(5-6), 337–346. https://doi.org/10.1007/s11106-021-00248-
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

