MODELING OF COMPLEXES OF LOW-BASIC ALUMINUM OXYCHLORIDE WITH ORTHOSILICATE ACIDS IN AQUEOUS SOLUTION
DOI:
https://doi.org/10.15421/jchemtech.v31i1.271537Keywords:
orthosilicic acid, low-basic aluminum oxychloride, complexes, quantum chemical modeling, binding energy, Gibbs free energy, molecular spectrophotometryAbstract
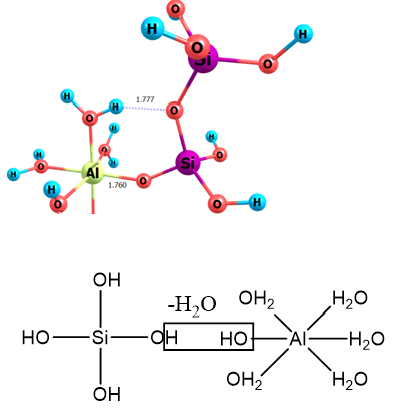
In the article, it is theoretically shown and experimentally confirmed that aluminum oxychloride "Alumofloc" at pH 7.5 interacts better with the monomeric form of orthosilicate acid. Determination of orthosilicic acid was carried out by the method of molecular spectrophotometry. Sodium fluorosilicate was used as a source of orthosilicic acid. With the use of quantum-chemical methods of research, the energetics of the interaction of aluminum oxychloride with orthosilicate acids was shown. The peculiarities of the structure of the formed complexes of the general composition [Al(H2O)5–L]2+ and [Al(OH)(H2O)4–L]+, where L is a monomeric, dimeric, and trimerous forms of orthosilicate acid, were established, as well as the energetics of the bonds between the central atom and ligands in the studied complexes were shown. Mechanisms of the formation of such complexes as [Al(H2O)5–OSi(OH)3]2+, [Al(H2O)5–OSi2O(OH)5]2+ and [Al(H2O)5–OSi3O2(OH)7]2+ were proposed, and their energy of formation was calculated. Furthermore, it was also noted that the nature of orthosilicate acid does not affect the binding energy of Aluminum with Oxygen in the complexes.
References
Igarashi, M., Matsumoto, T., Yagihashi, F., Yamashita, H., Ohhara, T., Hanashima, T., Nakao, F., Moyoshi, T., Sato, K., Shimada, S. (2017). Non-aqueous selective synthesis of orthosilicic acid and its oligomers. Nature communications, 8(1), 1–8.
https://doi.org/10.1038/s41467-017-00168-5
Potapov, V. V., Cerdan, A. A., Gorev, D. S. (2022). Silicic Acid Polymerization and SiO2 Nanoparticle Growth in Hydrothermal Solution. Polymers, 14(19), 4044. https://doi.org/10.3390/polym14194044
Pasenko, O., Mandryka, A., Khrupchyk, Y., Vereshchak, V. (2022). Stable solutions of orthosilicic acid. Voprosy khimii i khimicheskoi tekhnologii, 4, 56–60. https://www.doi.org/10.32434/0321-4095-2022-143-4-56-60
Li, S., Kang, Y. (2022). Impacts of key preparation factors on polymerization and flocculation performance of polyferric silicate sulfate (PFSiS). Colloids and Surfaces A: Physicochemical and Engineering Aspects, 635, 128109.https://doi.org/10.1016/j.colsurfa.2021.128109
Shestopalov, O. V., Getta, O. S., Rykusova, N. I. (2019). [Modern methods of wastewater treatment of the food industry]. Scientific and practical journal, Environmental Sciences, 2(25) 20–27. (In Ukrainian) https://doi.org/10.32846/2306-9716-2019-2-25-4
Frisch, M. J. E. A., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., ... & Fox, A. D. (2009). Gaussian 09, Revision D.01. Gaussian. Inc., Wallingford.
König, F. B., Schönbohm, J., Bayles, D. (2001). AIM2000-a program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Becke, A. D. (1993). Density-Functional Thermochemistry. III. The Role of Exact Exchange.Indian Journal of Pure & Applied Physics, 98(7), 5648–5656. https://doi.org/10.1063/1.464913
Lee, C., Yang, W., Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical review B, 37(2), 785. https://doi.org/10.1103/PhysRevB.37.785
Kravchenko, A. A., Demianenko, E. M., Grebenyuk, A. G., Terets, M. I., Portna, M. G., Lobanov, V. V. (2021). Quantum chemical study on the interaction of arginine with silica surface. Chemistry, Physics and Technology of Surface, 12(4), 358–364.
https://doi.org/10.15407/hftp12.04.358
Wiberg, K. B. (2004). Basis set effects on calculated geometries: 6‐311++G** vs. aug‐cc‐pVDZ. Journal of computational chemistry, 25(11), 1342–1346. https://doi.org/10.1002/jcc.20058
Demianenko, E., Ilchenko, M., Grebenyuk, A., Lobanov, V. (2011). A theoretical study on orthosilicic acid dissociation in water clusters. Chemical Physics Letters, 515(4–6), 274–277.
https://doi.org/10.1016/j.cplett.2011.09.038
Nimoth, J. P., Müller, T. (2021). The influence of ring strain on the formation of Si–H–Si stabilised oligosilanylsilyl cations. Dalton Transactions, 50(45), 16509–16513. https://doi.org/10.1039/D1DT03375A
Afshari, T., Mohsennia, M. (2019). Effect of the Si, Al and B doping on the sensing behaviour of carbon nanotubes toward ethylene oxide: a computational study. Molecular Simulation, 45(16), 1384–1394. https://doi.org/10.1080/08927022.2019.1635693
Willcox, D. R., De Rosa, D. M., Howley, J., Levy, A., Steven, A., Nichol, G. S., Morrison, C. A., Cowley, M. J., Thomas, S. P. (2021). Aluminium‐Catalyzed C(sp)−H Borylation of Alkynes. Angewandte Chemie International Edition, 60(38), 20672–20677. https://doi.org/10.1002/anie.202106216
Barone, V., Cossi, M., Tomasi, J. (1998). Geometry optimization of molecular structures in solution by the polarizable continuum model. Journal of Computational Chemistry, 19(4), 404–417.
https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Tomasi, J., Mennucci, B., Cammi, R. (2005). Quantum mechanical continuum solvation models.Chemicalreviews, 105(8), 2999–3094. https://doi.org/10.1021/cr9904009
Wick, C. R., Clark, T. (2018). On bond-critical points in QTAIM and weak interactions. Journal of Molecular Modeling, 24(6), 1–9. https://doi.org/10.1007/s00894-018-3684-x
Espinosa, E., Molins, E., Lecomte, C. (1998). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters, 285(3/4), 170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A. (2020). Formation of the π-complexes of copper atoms with acrylic, maleic and fumaric acids in aqueous medium. Journal of Chemistry and Technologies, 28(2), 153–116. http://dx.doi.org/10.15421/082016
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A., Glushkov, V. N. (2019). Features of (dπ-pπ)-binding of Cu(I) ions with acrylic, maleic and fumaric acids in aqueous solution. Journal of Chemistry and Technologies, 27(2), 148–157. https://doi.org/10.15421/081916
Bine, F. K., Tasheh, S. N., Nkungli, N. K. (2020). Corrosion Inhibition of Aluminium in Gas and Acid Media by Some Chalcone-Based N-(3-Aminopropyl) Imidazoles: TD-DFT-Based FMO, Conceptual DFT, QTAIM and EDA Studies. Computational Chemistry, 9(1), 37–63. https://doi.org/10.4236/cc.2021.91003
Álvarez‐Miguel, L., Burgoa, J. D., Mosquera, M. E., Hamilton, A., Whiteoak, C. J. (2021). Catalytic Formation of Cyclic Carbonates using Gallium Aminotrisphenolate Compounds and Comparison to their Aluminium Congeners: A Combined Experimental and Computational Study. ChemCatChem, 13(19), 4099–4110. https://doi.org/10.1002/cctc.202100910
Mandryka, A. G., Pasenko, O. O., Vereschak, V. H., Osokin, Y. S. (2022). Quantum chemical modeling of orthosilicic acid clusters with some acids in aqueous solution. Journal of Chemistry and Technologies, 30(2), 159–165. https://doi.org/10.15421/jchemtech.v30i2.258938
Mandryka, A. G., Pasenko, O. O., Vereschak, V. H., Osokin, Y. S. (2022). Quantum-chemical modeling of reactions involving low-base aluminum oxychloride with orthosilicate acid. Priority areas of research in scientific and educational activities: Problems and prospects, 273–275. (in Ukrainian) https://elartu.tntu.edu.ua/bitstream/lib/39056/1/%D0%97%D0%B1%D1%96%D1%80%D0%BD%D0%B8%D0%BA%202022.pdf#page=273
Kambalina, M., Mazurova, I., Skvortsova, L., Guseva, N., An, V. (2014). Study of aqueous chemical forms of silicon in organic-rich waters. Procedia Chemistry, 10, 36–42. https://doi.org/10.1016/j.proche.2014.10.008
Strickland, J. D. H. (1952). The preparation and properties of silicomolybdic acid. J. Amer. Chem. Soc., 74(4), 862–867. https://doi.org/10.1021/ja01124a002
Thomas, A. W., & Whitehead, T. H. (2002). Ion interchanges in aluminum oxychloride hydrosols. The Journal of Physical Chemistry, 35(1), 27–47. https://doi.org/10.1021/j150319a002
Gout, R., Pokrovski, G. S., Schott, J., Zwick, A. (2000). Raman spectroscopic study of aluminum silicate complexes at 20 C in basic solutions. Journal of solution chemistry, 29(12), 1173–1186. https://doi.org/10.1023/A:1026428027101
Applin, K. R. (1987). The diffusion of dissolved silica in dilute aqueous solution. Geochimica et Cosmochimica Acta, 51(8), 2147–2151.
https://doi.org/10.1016/0016-7037(87)90263-8
Swaddle, T. W. (2001). Silicate complexes of aluminum(III) in aqueous systems. Coordination Chemistry Reviews, 219, 665–686.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

