PHYSICOCHEMICAL CHARACTERISTICS OF THE HMTA – HCl – H2O BUFFER SYSTEM
DOI:
https://doi.org/10.15421/jchemtech.v31i1.273194Keywords:
hexamethylenetetramine; stability; hydrolysis; buffer system; buffer capacityAbstract
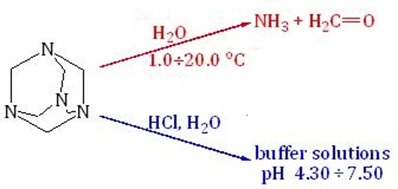
In the current study stability of hexamethylenetetramine (HMTA) aqueous solutions without acid additives (HCl, H2SO4, citrate-phosphate buffer systems, etc.) in the range of temperatures 1.0 ÷ 20.0 °С and concentrations of HMTA 0.10 ÷ 1.0 M (pH0 = 7.05 ÷ 8.25) was investigated by the spectrophotometric and potentiometric (pH and redox) methods. The obtained data indicate the complexity of the mechanism of hydrolytic transformations and acid-base interactions in the studied HMTA solutions. It was noted that the mechanism of disproportionation of HMTA in water significantly depends on temperature and its concentration. The contents of ammonium ions and formaldehyde as the final products of HMTA hydrolysis was determinated. The molar concentration of ammonium ions is no more than 5.0 % of the total content of HMTA and is several times higher than the formaldehyde concentration. It is shown that the dynamics of ammonium ions and formaldehyde accumulation does not correlate with the potentiometric curves. It was established that hydrolytic stability of HMTA aqueous solutions increases with it concentration. Recommendations for the preparation of buffer solutions based on HMTA are formulated. It is noted that it is desirable to prepare aqueous HMTA solutions of high concentration (³ 0.50 M) and store them at room temperature. An algebraic equation that describes the ratio of volumes of solutions of 1.00 M (2.50 M) HMTA and 0.10 M HCl, which are required for the preparation of buffer solutions with a certain pH in the range of 4.30 ÷ 7.40 (5.00 ÷ 7.50) was obtained. Mathematical model that describes the concentration dependence of the HMTA – HCl – H2O buffer solutions capacity and the influence of dilution on the change in pH has been proposed. It is noted that the buffer capacity of the studied buffer system depends significantly on the concentration and ratio of the components in the solution.
References
Eller, K., Henkes, E., Rossbacher, R., Höke, H. (2005). Amines, Aliphatic. in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim. http://dx.doi.org/10.1002/14356007.a02_001
Kaur, N., Kishore, D. (2013) An insight into hexamethylenetetramine: a versatile reagent in organic synthesis. J. Iran. Chem. Soc. 10, 1193–1228. http://dx.doi.org/10.1007/s13738-013-0260-2
Ennan, A. A.-A., Khoma, R. E., Dlubovskii, R. M., Zukharenko, Yu. S., Bienkovska, T.S., Knysh, I. M. (2022) Mono- and bifunctional impregnated fiber chemosorbents for respiratory purpose. Visn. Odes. nac. univ., Him. 27(1), 6–36. (in Ukrainian). http://dx.doi.org/10.18524/2304-0947.2021.4(80).248297
Khoma, R. E., Shestaka, A. A., Shishkin, O. V., Baumer, V. N., Brusilovskii, Yu. E., Koroeva, L. V., Ennan, A. A., Gelmboldt, V. O. (2011). Features of interaction in the sulfur(IV) oxide-hexamethylenetetramine-water system: A first example of identification of the product with a sulfur-carbon bond. Russ. J. Gen. Chem., 81(3), 620–621. http://dx.doi.org/10.1134/S1070363211030352
Altinoz, M. A., Ozpinar, A., Ozpinar, A., Perez, J. L., Elmaci, İ. (2019). Methenamine's journey of 160 years: Repurposal of an old urinary antiseptic for treatment and hypoxic radiosensitization of cancers and glioblastoma. Clin. Exp. Pharm. Physiol., 46(5), 407–412. http://dx.doi.org/10.1111/1440-1681.13070
Shokrollahi, A., Ghaedi, M., Niband, M. S., Rajabi, H. R. (2008). Selective and sensitive spectrophotometric method for determination of sub-micro-molar amounts of aluminium ion. J. Hazard. Mater. 151(2–3), 642–648. http://dx.doi.org/10.1016/j.jhazmat.2007.06.037
Kumar, S., Goswami, A. K., Purohit, D. N. (2003). A Review of Hydroxytriazenes. Rev. Anal. Chem. 22(1), 73-80. http://dx.doi.org/10.1515/REVAC.2003.22.1.73
Amin, A. S., Kassem, M. A., Mohammed, T. Y. (2015). Utilization of cloud-point extraction for colorimetric determination of trace amounts of thorium(IV) in real samples. RSC Adv. 5(64), 52095–52100. http://dx.doi.org/10.1039/C5RA08806B
Liu, Q., Yang, Y. C., Chen, N. H., Li, Y. M. (2018). Determination of Lead (II) in environmental water samples by resonance light scattering technology. IOP Conf. Ser. Mater. Sci. Eng. 392, 062099. http://dx.doi.org/10.1088/1757-899X/392/6/062099
Madrakian, T., Afkhami, A., Mousavi, A. (2007). Spectrophotometric determination of trace amounts of uranium(VI) in water samples after mixed micelle-mediated extraction. Talanta. 71(2), 610–614. http://dx.doi.org/10.1016/j.talanta.2006.05.002
Chebotarev, A. N. (2013). Composition and relative stability of ion-molecular forms that are realized in the system water – tetrafluoroboric acid – hexamethylenetetramine. Visn. Odes. nac. univ., Him., 18(3) 79–88. http://dx.doi.org/10.18524/2304-0947.2013.3(47).31177 (in Russian)
Ombaka, O. (2020). Complexometric determination of zinc using hydroxytriazene as a metallochromic indicator. Res. J. Life Sci. Bioinf. Pharm. Chem. Sci. 6(5), 77–88. http://dx.doi.org/10.26479/2020.0605.07
Ruxiang, S., Li, Z., Shulian, C. (2017) Determination of high content cadmium in tin-lead solder by EDTA titration. Metallurg. Anal. 37(10), 79–83. http://dx.doi.org/10.13228/j.boyuan.issn1000-7571.010134
Chen, X., Cai, C., Luo, H., Zhang, G. (2005). Study on the resonance light-scattering spec-trum of anionic dye xylenol orange-cetyltrimethylammonium-nucleic acids system and determination of nucleic acids at nanogram levels. Spectrochim. Acta Part A. 61(9), 2215–2220. http://dx.doi.org/10.1016/j.saa.2004.08.023
Sife-Eldeen, Kh. A. (2013). Tracing of γ-radiation-induced electrical conductivity and pH change of hexamethylenetetramine aqueous solutions and its applications. Appl. Radiat. Isotop., 74, 152–156. http://dx.doi.org/10.1016/j.apradiso.2012.12.004
Strom Jr., J. G., Won Jun, H. (1980). Kinetics of hydrolysis of methenamine. Pharm. Bull. 69(11), 1261–1263. http://dx.doi.org/10.1002/jps.2600691107
Hutnan, M., Drtil, M., Derco, J., Mrafkova, L. (2005). Biodegradation of Hexamethylenetetramine in Anaerobic Baffled Reactor. Pol. J. Environ. Stud. 14(5), 585–591.
Takayanagi, T., Shimakami, N., Kurashina, M., Mizuguchi, H., Yabutani, T. (2016) Determination of the Acid-Base Dissociation Constant of Acid-Degradable Hexamethylenetetramine by Capillary Zone Electrophoresis. Anal. Sci. 32(12), 1327–1332. http://dx.doi.org/10.2116/analsci.32.1327
Novikov, Ju. V., Lastochkina, K. O., Boldina, Z. N. (1990). Metody issledovanija kachestva vody vodoemov. Moscow, Medicina. (in Russian)
Sasongko, A., Nugroho, R. W., Mulyani, D. (2018). Ammonia determination in bottled water using spectrophotometer: comparison between Nessler and Berthelot methods. J. Sains Teknol. 7(1), 126–134. http://dx.doi.org/10.23887/jstundiksha.v7i1.13009
Gigante, A. C., Gotardo, M. A., Tognolli, J. O., Pezza, L., Pezza, H. R. (2004). Spectrophotometric determination of formaldehyde with chromotropic acid in phosphoric acid medium assisted by microwave oven. Microchem. J. 77(1), 47–51. http://dx.doi.org/10.1016/j.microc.2003.12.002
Khoma, R.E., Gavrilenko, M.I. (2010). Anionic complexes as products of reactions in SO2 – carbamide(acetamide) – H2O system. Russ. J. Gen. Chem. 10(5), 899–904. https://doi.org/10.1134/s1070363210050051
Khoma R.E., Shestaka A.A., Gavrilenko M.I., Sokhranenko G.P., Gelmboldt V.O. (2011). Complexing of Sulfur(IV) Oxide with Hexamethylenetetramine and Hexamethylenediamine in Aqueous Solutions. Russ. J. Appl. Chem., 84(1), 17–24. https://doi.org/10.1134/s1070427211010034
Khoma R.E., Ennan A.A., Dlubovskii R.M., Ishkov Yu.V., Bienkovska T.S., Rakhlitskaya E.M. (2021). Equilibrium Processes in AlkNHCH2SO3H–NH2CH2CH2OH–H2O Solutions. Russ. J. Gen. Chem. 91(4), 583–592. https://doi.org/10.1134/s1070363221040010
Vinogradoff V., Rimola A., Duvernay F., Danger G., Theulé P., Chiavassa T. (2012). The mechanism of hexamethylenetetramine (HMT) formation in the solid state at low temperature. Phys. Chem. Chem. Phys. 14(35), 12309–12320. https://doi.org/10.1039/c2cp41963g
Urbansky, E. T., Schock, M. R. (2000). Understanding, Deriving, and Computing Buffer Capacity. J. Chem. Educ. 77(12), 1640–1644. http://dx.doi.org/10.1021/ed077p1640
Carlsson, A.-C. C., Veiga, A. X., Erdélyi, M. (2014). Halogen Bonding in Solution. In: Metrangolo, P., Resnati, G. (Eds), Halogen Bonding II. Topics in Current Chemistry, 359, 49–67. https://doi.org/10.1007/128_2014_607
Chettiyankandy, P., Chand, A., Ghosh, R., Sarkar, S. K., Das, P., Chowdhuri, S. (2019). Effects of hexamethylenetetramine (HMTA) on the aqueous solution structure, dynamics and ion solvation scenario: A concentration and temperature dependent study with potential HMTA models. J. Mol. Liq. 296, 111820. http://dx.doi.org/10.1016/j.molliq.2019.111820
Aladko, L. S., Komarov, V. Yu., Manakov, A. Yu., Ancharov A. I. (2007). Phase diagram of the hexamethylenetetramine: water system. J. Incl. Phenom. Macrocycl. Chem. 59(3-4), 389–391. http://dx.doi.org/10.1007/s10847-007-9342-z
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

