THE 4-HALOGENOPHENYLGLYOXALS INTERACTION WITH N-ALKOXY-N’-ARYLUREAS
DOI:
https://doi.org/10.15421/jchemtech.v31i1.273820Keywords:
arylglyoxals; N-alkoxy-N’-arylureas; synthesis; 3-alkoxy-5-aryl-4,5-dihydroxy-1-phenylimidazolidin-2-ones; 3-alkoxy-5-aryl-1-phenylimidazolidine-2,4-diones.Abstract
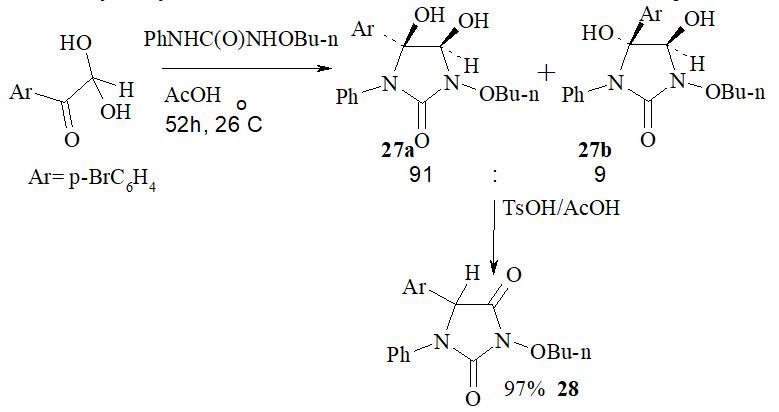
Aim. To investigate the 4-X-phenylglyoxal (X = F, Cl, Br) interaction with N-alkoxy-N’-arylureas in acetic acid medium at room temperature. Methods. Mass spectrometry, 1H and 13C NMR spectroscopy. Results. We have showed these reaction type peculiarities and several different tendencies depending on the aryl glyoxal moiety structure. In this article we have described the influence of this moiety at the stage of the hydantoin formation. It has been found that the 4-fluorophenylglyoxal hydrate interacts with N-methoxy-N’-phenylurea in acetic acid during 99 h at 26 °C yielding the mixture of 5-(4-fluorophenyl)-cis-4,5-dihydroxy-3-methoxy-1-phenylimidazolidin-2-one, 5-(4-fluorophenyl)-trans-4,5-dihydroxy-3-methoxy-1-phenylimidazolidin-2-one, and 5-(4-fluorophenyl)-3-methoxy-1-phenylimidazolidine-2,4-dione. The 5-(4-fluorophenyl)-3-methoxy-1-phenylimidazolidine-2,4-dione is yielded because of the additional exposure the products mixture in acetic acid during 260 h. The 4-chlorophenylglyoxal hydrate reacts with N-ethoxy-N’-phenylurea in acetic acid at 26–27 °C during 219 h yielding the mixture of 5-(4-chlorophenyl)-3-ethoxy-cis-4,5-dihydroxy-1-phenylimidazolidin-2-one and 5-(4-chlorophenyl)-3-ethoxy-1-phenylimidazolidine-2,4-dione. After being processed by the p-toluenesulfonic acid this mixture is converted in pure 5-(4-chlorophenyl)-3-ethoxy-1-phenylimidazolidine-2,4-dione. 4-Bromophenylglyoxal hydrate interacts with N-n-butyloxy-N’-phenylurea in acetic acid during 52 h at 26 °C yielding only the mixture of the diastereomers of 5-(4-bromophenyl)-3-n-butyloxy-4,5-dihydroxy-1-phenylimidazolidin-2-one. The molar ratio of 5-(4-bromophenyl)-3-n-butyloxy-cis-4,5-dihydroxy-1-phenylimidazolidin-2-one and 5-(4-bromophenyl)-3-n-butyloxy-trans-4,5-dihydroxy-1-phenylimidazolidin-2-one is 91 : 9. The diastereomers of 5-(4-bromophenyl)-3-n-butyloxy-4,5-dihydroxy-1-phenylimidazolidin-2-one were converted into 5-(4-bromophenyl)-3-n-butyloxy-1-phenylimidazolidine-2,4-dione because of the p-toluenesulfonic acid action. As a conclusion we have proposed the possible mechanism of 3-alkoxy-5-aryl-4,5-dihydroxy-1-phenylimidazolidin-2-ones conversion into 3-alkoxy-5-aryl-1-phenylhydantoins. Conclusions. It has been found that the 4-halogenophenylglyoxal interaction with N-alkoxy-N’-phenylureas in acetic acid at room temperature under the further processing by of p-toluenesulfonic acid is new way to synthetize 3-alkoxy-5-(4-halohenophenyl)-1-phenylhydantoins. It has been shown that there is a certain tendency depending on the 4-halogenophenyl substituent nature of the influence on the reaction.
References
Meusel, M., Gutschow, M. (2004). Recent Developmments in Hydantoin Chemistry. A Review. Org. Prep.Proced. Int., 36(5), 391–443.https://doi.org/10.1080/00304940409356627
Yang, C.; Schanne, F.A.X.; Yoganathan, S.; Stephani, R.A. (2016). Synthesis os N-1’,N-3’-disubstituted spirohydantoins and their anticonvulsant activities in pilocarpine model of temporal lobe epipepsy. Bioorg. Med. Chem. Lett., 26(12), 2912–2914. http:// doi.org/10.1016/j.bmcl.2016.04.040
Sadarangani, I.R., Bhatia, S., Amarante, D., Lengyel, I., Stephani, R.A. (2012). Synthesis, resolution and anticonvulsant activity of chiral N-1’-ethyl,N-3’-(1-phenylethyl)-(R,S)-2’H,3H,5’H-spiro-(2-benzofuran-1.4’-imidazolidine)-2’,3,5’-trione diastereomers. Bioorg. Med. Chem. Lett., 22(7), 2507–2509. http:// doi.org/10.1016/j.bmcl.2012.02.005
Konnert, L., Lamaty, F., Martinez, J., Colacino, E. (2017). Recent Advances in the Synthesis of Hydantoins: The State of the Art of Valuable Scaffold, Chem. Rev., 117(23), 13757–13809; doi: 10.1021/acs.chemrev.7b00067.
Hulme, C., Ma., L., Romano, J., Morton, J., Tang, S-Y., Cherrier, M.-P., Choi, S., Salvino, J., Labaudiniere, R. (2000). Novel Applications of Carbon Dioxide|MeOH for the Synthesis of Hydantoins and Cyclic Ureas Via the Ugi Reactions. Tetrahedron Lett., 41, 1889–1893.
Lengyel, I.; Patel, H.J.; Stephani, R.A. (2007). The preparation and characterization of ninetheen new phtalidyl spirohydantoins. Heterocycles., 73, 349–375.
Patel, H.J., Sarra, J., Caruso, F., Rossi, M., Doshi, U., Stephani, R.A. (2006). Synthesis and anticonvulsant activity of new N-1’,N-3’-disubstituted-2’H,3H,5’H-spiro-(2-benzofuran-1,4’-imidazolidine)-2’,3,5’-triones. Bioorg.Med.Chem.Lett., 16(17), 4644–4647. doi:10.1016/j.bmcl.2006.05.102
Savjani, J. K., Gajjar, A.K. (2011). Pharmauceutical importance and Synthethic Strategies for imidazolidin-2-thione and Imidazol-2-thione Derivatives. Pak. J. Biol. Sci., 14, 1076–1089.
Staake, M.D., Kashinatham, A., McMorris, T.C., Estes, L.A., Kelner, M.J. (2016). Hydroxyurea derivatives of irofulven with improved antitumor efficacy. Bioorg. Med. Chem. Lett., 26(7), 1836–1838. doi:10.1016/j.bmcl.2016.02.028
Eftekhari-Sis, B., Zirak, M., Akrabi, A. (2013). Arylglyoxals in Synthesis of Heterocyclic Compounds, Chem. Rev., 113(5), 2958–3043; DOI: 10.1021/cr300176g
Shtamburg, V.G., Anishchenko, A.A., Shtamburg, V.V., Shishkin, O.V., Zubatyuk, R.I., Mazepa, A.V., Rakipov, I.M., Kostyanovsky, R.G. (2008). Synthesis and crystal structure of new imidazolidine-2,4-dione and imidazolidin-2-one derivatives, Mendeleev Commun., 18, 102–104. doi: 10.1016/j.mencom.2008.03.018.
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Zubatyuk R.I., Mazepa, A.V., Klotz, E.A., Kravchenko, S.V., Kostyanovsky, R.G. (2015). Single-stage synthesis of 3-hydroxy- and 3-alkoxy-5-arylimidazolidine-2,4-diones by reaction of arylglyoxal hydrates with N-hydroxy- and N-alkoxyureas, Chem. Heterocycl. Comp., 51(6), 553–559. doi 10.1007/s10593-015-1735-0.
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Shishkina, S.V., Mazepa, A.V., Konovalova, I.S. (2020). Interactions of Ninhydrin with N-Hydroxyurea and N-Alkoxyureas in Acetic Acid. Eur. Chem. Bull., 9(5), 125–131. http:/dx.doi.org/10.17628/ecb.2020.9.125-131
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Rusanov, E.B., Kravchenko, S.V., Mazepa, A.V. (2021). The Interaction of the 4-Carboxyphenylglyoxal with N-Hydroxyurea and N-Alkoxy-N’-alkyl(aryl)ureas. The Structure of 5-(Carboxyphenyl)-4,5-dihydroxy-1-methyl-3-propyloxyimidazolidin-2-one. Journal of Chemistry and Technologies, 29(4), 518–527; doi: 10.15421/jchemtech.v29i4.233171
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A. (2020). Mazepa, A.V. The peculiarities of the 4-Carboxyphenylglyoxal and N-Alkoxy-N’-arylureas interaction. Eur. Chem. Bull., 9(11), 339–344, http:/dx.doi.org/10.17628/ecb.2020.9.339-344
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A. A., Mazepa, A.V., Rusanov, E.B. (2022). 3-Alkoxy-1,5-bis(aryl)imidazolidine-2,4-diones, synthesis and structure. J. Mol. Struct., 1264, 133259; https://doi.org/10.1016/j.molstruc.2022.133259
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Shishkina, S.V., Mazepa, A.V., Konovalova, I.S. (2019). 3-Alkoxy-1,5-diaryl-4,5-dihydroxyimidazolidin-2-ones and 3-Alkoxy-1-alkyl-5-aryl-4,5-dihydroxyimidazo-lidin-2-ones: Synthesis and Structure. Eur. Chem. Bull., 8(9), 282–290. http:/dx.doi.org./10.17628/ecb.2019.8.282-290.
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Mazepa, A.V., Shishkina, I.S., Konovalova, S.V. (2019). Synthesis and structure of 3,4,5-trihydroxy-5-(4-nitrophenyl)imidazolidin-2-one. Eur. Chem. Bull., 8(4), 110–114, doi: 10.17628/ecb.2019.8.110-114
Kostyanovsky, R.G., Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Shtamburg, V.V., Anishchenko, A.A., Mazepa, A.V. (2010). Pyramidal nitrogen in the crystal of N-[(benzoyl)-(hydroxy)methyl]-N-benzyloxy-N’-(2-bromophenyl)urea. Mendeleev Commun., 20(3), 167–169, https://doi.org/10.1016/j.mencom.2010.05.015
Shtamburg, V.G., Shtamburg, V.V., Anishchenko, A.A., Rusanov, E.B., Kravchenko, S.V. (2021). The Structure of 1-Ethoxy-3a,8a-dihydroxy-3-(1-naphthyl)methyl-1,3,3a,8a-tetrahydroindeno[1,2-d]imidazole-2,8-dione. Journal of Chemistry and Technologies, 29(2), 232–239, https://doi.org/10.15421/jchemtech.v29i2.231195
Shtamburg, V.G., Anishchenko, A.A., Shtamburg, V.V., Pletenez, A.V., Zubatyuk, R.I., Shishkin, O.V. (2011). Synthesis and structure of N-[(benzoyl)-(hydroxy)methyl]-N-ethoxy-N’-(2-bromophenyl)urea. Voprosy Khimii i Khim. Technology, (5), 13–17 (In Russian).
Perronnet, J., Demoute, J.P. (1982). Approach to the 1-methoxy-2-benzimidazolinones. Gazz. Chim. Ital., 112, 507–511.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

