ISOTHERMAL SECTION FOR THE TERNARY CeO2-Lа2O3-Dy2O3 SYSTEM AT 1100 °С
DOI:
https://doi.org/10.15421/jchemtech.v31i2.275434Keywords:
phase equilibria, phase diagram, solid solution, lattice parameters, functional ceramicsAbstract
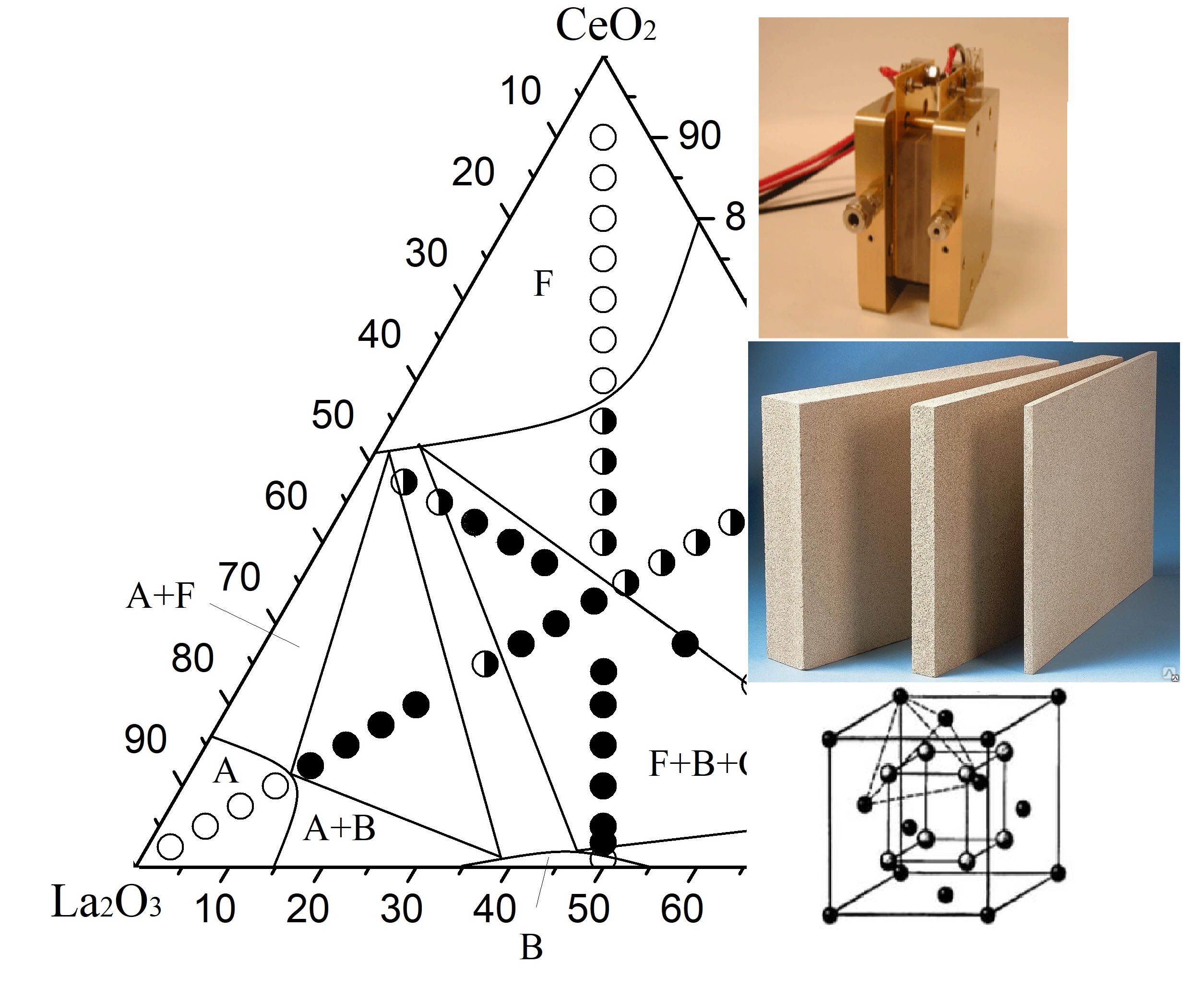
Using recently published scientific literature, been being provided that scientists around the world have grown increasingly interested in materials based on cerium oxide doped with rare earth metal oxides. The undeniable fact of that the study of phase equilibria of multicomponent oxide systems is both the physical and chemical basis for novel improved materials design. Among the important tasks in the study of phase equilibria of multicomponent systems is to determine the stability limits of solid solutions in a certain temperature and concentration range as well as to confirm the existence of ordered phases. In the present work, the phase equilibria of the ternary system CeO2-La2O3-Dy2O3 were investigated in the whole concentration range. The performed work was to construct an isothermal cross-section of the phase diagram CeO2-La2O3-Dy2O3 ternary system at 1100 °C. The obtained results indicate the absence of the formation of new phases in the studied system under the used technological conditions. By the method of XRD, it was determined that the formation of solid solutions based on the (F) modification of CeO2 with a fluorite-type structure, as well as monoclinic (B) and hexagonal (A) modifications of rare earth oxides, is observed in the studied system. The values of the lattice unit cell parameters of solid solutions formed in the ternary CeO2-La2O3-Dy2O3 system at a temperature of 1100 °C were analyzed. From the obtained data, it can be concluded that the lattice unit cell parameters of cubic solid solutions formed in the studied system change linearly following Wegard's law. The formation of a cubic solid solution with a fluorite-type structure F-CeO2 results in the replacement of tetravalent Ce4+ ions with trivalent Ln3+ ions. As a result, there is an increase in the unit cell parameter for cubic solid solutions with a fluorite-type structure, since the replacement occurs with ions with a larger ionic radius.
References
Polychronopoulou, K., Dabbawala, A. A., Sajjad, M., Singh, N., Anjum, D. H., Baker, M. A., Charisiou, N.D., Goula, M.A. (2022). Hydrogen production via steam reforming of glycerol over Ce-La-Cu-O ternary oxide catalyst: An experimental and DFT study. Applied Surface Science, 586, 152798. https://doi.org/10.1016/j.apsusc.2022.152798
Siakavelas G. I., Charisiou, N. D., lKhoorid, A. A., Sebastian, V., Hinder, S. J., Baker, M. A., Yentekaki, I. V., Polychronopoulou, K., Goula, M. A. (2022). Cerium oxide catalysts for oxidative coupling of methane reaction: Effect of lithium, samarium and lanthanum dopants. Journal of Environmental Chemical Engineering, 10(2), 107259. https://doi.org/10.1016/j.jece.2022.107259
Zinatloo-Ajabshir, S. (2022). 3-Rare earth cerate (Re2Ce2O7) ceramic nanomaterials. Advanced Rare. Earth-Based Ceramic Nanomaterials, 47–54. https://doi.org/10.1016/B978-0-323-89957-4.00009-8
Zeng, S., Wang, Z., Xitang, Y. M., Hao, W., Yunji, L., Yueying, D., Qiao, L. E., Qian, W. (2022). Effect of lanthanum content on the thermophysical properties and near-infrared reflection properties of lanthanum-cerium oxides. Solid State Sciences, 124, 106805. https://doi.org/10.1016/j.solidstatesciences.2021.106805
Aponte, Á. G., Ramírez, M. A L., Mora, Y. C., Marín, J. F S., Sierra, R. B. (2020). Cerium oxide nanoparticles for color removal of indigo carmine and methylene blue solutions. AIMS Materials Science, 7(4), 468–485. https://doi.org/10.3934/matersci.2020.4.468
Yang, C., Lu, Y., Zhang, L., Kong, Z., Yang, T., Tao, L., Zou, Y., Wang, S. (2021). Defect Engineering on CeO2-Based Catalysts for Heterogeneous Catalytic Applications. Small Strictures, 2(12), 2100058. https://doi.org/10.1002/sstr.202100058
Lu, G., Zheng, H., Lv, J., Wang, G., Huang, X. (2020). Review of recent research work on CeO2-based electrocatalysts in liquid-phase electrolytes. Journal of Power Sources, 480, 229091. https://doi.org/10.1016/j.jpowsour.2020.229091
Li, T., Tsyshevsky, R., McEntee, M., Durke, E. M., Karwacki, C., Rodriguez, E. E., Kuklja, M. M. (2022). Aliovalent-Doping Effects on the Surface Activity of Mesoporous CeO2 toward Nerve Agent Simulant DMMP Decomposition. The Journal of Physical Chemistry C, 126(42), 17923–17934. https://doi.org/10.1021/acs.jpcc.2c04853
Lavrynenko, O. M., Zahornyi, M. M., Vember, V. V., Pavlenko, O. Y., Lobunets, T. F., Kolomys, O. F., Povnitsa, O. Y., Artiukh, L. O., Naumenko, K. S., Zahorodnia, S. D., Garmasheva, I. L. (2022). Nanocomposites Based on Cerium, Lanthanum, and Titanium Oxides Doped with Silver for Biomedical Application. Condensed Matter, 7(3), 45. https://doi.org/10.3390/condmat7030045
Vember, V. V., Lavrynenko, O. M., Zahornyi, M. M., Pavlenko, O. Y., Benatov, D. E. (2022). Study of the Biological Activity of Nanoparticles of Lanthanum, Cerium and Titanium Oxides and their Composites Modified with Silver. Bulletin of NTUU "KPI named after Ihor Sikorskyi". Series: Chemical engineering, ecology and resource conservation, 2, 79–87. https://doi.org/10.20535/2617-9741.2.2022.260354
Asati, A., Santra, S., Kaittanis, Ch., Nath, S., Perez, J. M. (2009). Oxidase-Like Activity of Polymer-Coated Cerium Oxide Nanoparticles. Angewandte Chemie (International ed. in English), 48(13), 2308–2312. https://doi.org/10.1002/anie.200805279
Salehi, Z., Zinatloo-Ajabshir , S., Salavati-Niasari, M. (2017). Dysprosium cerate nanostructures: facile synthesis, characterization, optical and photocatalytic properties. Journal of rare earths, 35(8), 805–812. https://doi.org/10.1016/S1002-0721(17)60980-3
Andrievskaya, E. R., Kornienko, O. A., Sameljuk, A. V., Sayir, A. (2011). Phase Relation Studies in the CeO2-La2O3 System at 1100 to 1500 °С. Journal of the European Ceramic Society, 31(7), 1277–1283. https://doi.org/10.1016/j.jeurceramsoc.2010.05.024
Andrievskaya, E. R., Kornienko, O. A., Sayir, A., Vasylkiv, O. O., Sakka, Y. (2011). Phaserelation studies in the ZrO2–CeO2–La2O3system at 1500 °С. Journal of the American Ceramic Society, 94(6), 1911–1919. https://doi.org/10.1111/j.1551-2916.2010.04316.x
Коrnienko, О. А., Аndrievska, O. R. (2020). Phase equilibria in the Systems with ZrO2, CeO2 and Dy2O3. Innovative scientific researches: European development trends and regional aspect. Collective monograph, 4th ed, 155–179. https://doi.org/10.30525/978-9934-588-38-9
Grover, V., Tyagi, A. K. (2013). Ternary phase relations in CeO2–DyO1.5–ZrO2 system. Ceramics International, 39, 7563–7569. https://doi.org/10.1016/j.ceramint.2013.03.009
Minkova, N., Aslanian, S. (1989). Isomorphic substitutions in the CeO2-La2O3 system at 850 C. Crystal Research and Technology, 24(4), 351–354. https://doi.org/10.1002/crat.2170240402
Sibieude, F., Schiffmacher, G., Caro, P. (1978). Étude au microscope électronique de structures modulées dans les régions systéme La2O3-CeO2 riches en La2O3. Journal of Solid State Chemistry, 23(3-4), 361–367. https://doi.org/10.1016/0022-4596(78)90085-3
Kornienko, O., Bykov, O., Sameliuk, A., Yurchenko, Y. (2020). Phase relation studies in the CeO2-La2O3-Eu2O3 system at 1250 °С. Ukrainian Chemistry Journal, 86(3), 35–47. https://doi.org/10.33609/0041-6045.86.3.2020.35-47
Korniienko, O. A., Yushkevych, S. V., Bykov, O. I., Samelyuk, A. V., Bataiev, Yu. M., Zamula M. V. (2022). Phase equilibrium in binary La2O3-Dy2O3 and ternary CeO2-La2O3-Dy2O3 systems. Journal of the European Ceramic Society, 42(13), 5820–5830. https://doi.org/10.1016/j.jeurceramsoc.2022.06.045
Schneider, J., Matsuoka, M., Takeuchi, M., Zhang, J., Horiuchi, Y., Anpo, M., Bahnemann, D. W. (2014). Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chemical Reviews, 114(19), 9919–9986. https://doi.org/10.1021/cr5001892
Aponte, Á. G., Ramírez, M. A L., Mora, Y. C., Marín, J. F S., Sierra, R. B. (2020). Cerium oxide nanoparticles for color removal of indigo carmine and methylene blue solutions. AIMS Materials Science, 7(4), 468–485. https://doi.org/10.3934/matersci.2020.4.468
Xu, Y., Deng, Ch., Dong, Ch., Wang, Q., Gao, N. (2022). Synthesis and Oxygen Storage Capability of CeO2 Powders for Enhanced Photocatalytic Degradation of Acid Orange. International Journal of Photoenergy. https://doi.org/10.1155/2022/8594451
Lavrynenko, O. M., Zahornyi, M. M., Pavlenko, O. Yu., Bykov, A. I. (2022). Structure and thermal behavior of CeO2 and TiO2 nanopowders doped with noble metals. Applied Nanoscience. 13, 5115–5124. https://doi.org/10.1007/s13204-022-02706-0
Safari-Amiri, M., Mortazavi-Derazkola, S., Salavati Niasari, M., Ghoreishi, S. M. (2017). Synthesis and characterization of Dy2O3 nanostructures: enhanced photocatalytic degradation of rhodamine B under UV irradiation. Journal of Materials Science: Materials in Electronics. 28, 6467–6474 https://doi.org/10.1007/s10854-017-6333-8
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

