TECHNOLOGICAL PARAMETERS OF GALVANICHEMICAL PROCESSES OF FORMATION OF COBALT-BASED METAL OXIDE COMPOSITES
DOI:
https://doi.org/10.15421/jchemtech.v31i2.275741Keywords:
composite coatings; electrochemical deposition; cobalt, refractory metals; conductivity; scattering ability.Abstract
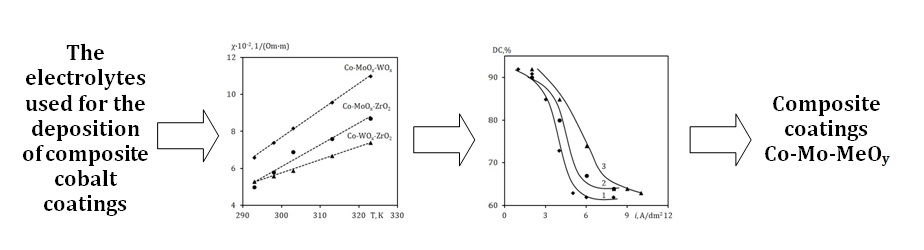
Electrodeposition of composite coatings based on cobalt, including refractory metals, allows obtaining coatings with a unique combination of physicochemical properties that cannot be achieved with other coating methods. The application of high-quality electrochemical composite coatings is only possible by establishing the characteristics of the electrolyte and electrolysis parameters. The characteristics of the scattering ability and specific electrical conductivity of Co-MoOx-WOx, Co-WOx-ZrO2, and Co-MoOx-ZrO2 electrolytes for depositing cobalt-based composite coatings have been established. The electrical conductivity values of the electrolytes for depositing cobalt-based composite coatings linearly increase with the temperature of the electrolyte, but their values at 25-30°C are sufficient for the deposition of high-quality coatings. It has been determined that within the current density range of 0.5–3.0 A/dm2 for Co-MoOx-WOx and up to 4.0 A/dm2 for Co-MoOx-ZrO2 and Co-WOx-ZrO2, the scattering ability remains above 85 %. The calculated activation energy of the electrical conductivity of complex electrolytes ranges from 22 to 29 kJ/mol, indicating the occurrence of mass transfer processes in the diffusion regime. Using insoluble inert stainless steel anodes in the technological process is justified.
References
Walsh, F. C., Larson, C. A. (2020). Towards improved electroplating of metal-particle composite coatings. The International Journal of Surface Engineering and Coatings, 98, 288–299. https://doi.org/10.1080/00202967.2020.1819022.
Wang, S., Walsh, F.C., Zhou, N. (2020). The electrodeposition of composite coatings: diversity, applications and challenges, Current Opinion in Electrochemistry, 20, 8–19. https://doi.org/10.1016/j.coelec.2020.01.011
Parshutin, V.V., Paramonov, A.M., Koval, A.V. (2022). Korrozionnyie i elektrohimicheskie svoystva splavov sistemyi Ni-Re, legirovannyih tsirkoniem, gafniem, volframom i palladium. Elektronnaya obrabotka materyialov, 58(4), 55–69. https://doi.org/10.52577/eom.2022.58.4.55
Sajjadnejad, M., Haghshenas, S., Tavakoli Targhi, V., Setoudeh, N., Hadipour, A., Moghanian, A., Hosseinpour, S. (2021). Wear behavior of alkaline pulsed electrodeposited nickel composite coatings reinforced by ZnO nanoparticles. Wear, 468–469, doi: 10.1016/j.wear.2020.203591.
Zanella, C., Lekka, M., Bonora, P. L. (2009). Influence of the particle size on the mechanical and electrochemical behaviour of microand nano-nickel matrix composite coatings. J. Appl. Electrochem., 39, 31–38, doi: 10.1007/s10800-008-9635-y
Ortolani, M., Zanella, C., Azanza Ricardo, C. L. (2012). Scardi Elastic grain interaction in electrodeposited nanocomposite nickel matrix coatings. Surface and Coatings Technology, 206, 2499–2505, doi: 10.1016/j.surfcoat.2011.10.056
Huang, J. M., Li, Y., Zhang, G. F., Hou, X. D. (2013). Deng electroplating of Ni-ZrO2 nanocomposite coatings on 40CrNiMo7 alloy. Surface Engineering, 29, 194–199, doi:10.1179/1743294412Y.0000000108
Wang, W., Hou, F.Y., Wang, H., Guo H. T. (2005). Fabrication and characterization of Ni-ZrO2 composite nano-coatings by pulse electrodeposition. Scripta Materialia, 53, 613–618, doi: 10.1016/j.scriptamat.2005.04.002
Sajjadnejad, M., Haghshenas, S.M.S, Tavakoli Targhi, V., Setoudeh, N., Hadipour, A., Moghanian, A., Hosseinpour S. (2021). Wear behavior of alkaline pulsed electrodeposited nickel composite coatings reinforced by ZnO nanoparticles. Wear, 468–469, 203591, doi:10.1016/j.wear.2020.203591
Nenastina, T.A., Sakhnenko, M.D., Proskurina, V.O. (2023) Use of materials based on cobalt alloys for the eco and energy technologies. Functional Materials, 30(1), 43–48. https://doi.org/10.15407/fm30.01.43
Low, C.T.J. Wills, R.G.A. Walsh F.C. (2006) Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surface and Coatings Technology, 201, 371–383, doi:10.1016/j.surfcoat.2005.11.123
Sknar, Y. E., Sknar, I. V., Savchuk, O. O., Hrydnieva, T. V., Butyrina, O. D. (2023) Electrodeposition of Ni-zirconia or Ni-titania composite coatings from a methanesulfonic acid bath. Surface and Coatings Technology, 452, 129120. https://doi.org/10.1016/j.surfcoat.2022.129120
Tsynsaru, N., Cesiulis, H., Donten, M., Sort, J., Pellicer, E., Podlaha-Murphy, E. (2012). Modern trends in tungsten alloys electrodeposition with iron group metals. Surface Engineering and Applied Electrochemistry, 48, 491 – 520. doi: 10.3103/S1068375512060038.
Eliaz, N. Gileadi, E. (2008) Induced codepositio of alloys of tungsten, molybdenum and rhenium with transition metals. Modern Aspects of Electrochemistry, 42, 191 – 301. doi: 10.1007/978-0-387-49489-0_4.
Yar-Mukhamedova, G. Sakhnenko, N. Nenastina, T. (2018). Electrodeposition and properties of binary and ternary cobalt alloys with molybdenum and tungsten. Applied Surface Science, 445, 298–307. doi: 10.1016/j.apsusc.2018.03.171.
Karakurkchi, A.V., Ved’, M.V., Ermolenko, I.Yu., Sakhnenko, N.D. (2016). Electrochemical Deposition of Fe–Mo–W Alloy Coatings from Citrate Electrolyte. Surface Engineering and Applied Electrochemistry, 52, 43–49. https://doi.org/10.3103/S1068375516010087
Nenastina, T.A., Ved’, M.V., Sakhnenko, N.D., Proskurina, V.O. (2021). Effect of Electrolysis Conditions on the Composition and Microhardness of Ternary Cobalt Alloy Coatings. Surface Engineering and Applied Electrochemistry, 57(1), 59–66. https://doi.org/10.3103/s1068375521010099
Nenastina, T.O., Ved, M.V., Sakhnenko, M.D., Dacenko, V.V., Lavrova, I.O. (2021). The synthesis and photocatalytic properties of the cobalt-based composites with refractory metals. Journal of Chemistry and Technologiesthis, 28(3), 260–268. doi: 10.15421/082028
Nenastina, T.A., Ved, M.V., Sakhnenko, N.D., Proskurina, V.O., Fomina, L.P. (2020). Galvanochemical formation of functional coatings by the cobalt-tungsten-zirconium alloys Functional Materials, 27(2), 348–353. doi.org/10.15407/fm27.02.348
Ohno, T. Sarukawa, K. Matsumura, M. (2006). Direct observation of suppressed recombination of electron-hole pairs in the TiO2 nanopowders with anatase-rutile interface: in-situ NEXAFS study under UV irradiation. The Journal of Physical Chemistry A, 105, 2417–2425. doi: 10.1109/NMDC.2006.4388962
Yu, J. Low, J. Xiao, W. Zhou, P. (2014). Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Co-exposed {001} and {101} Facets. Journal of the American Chemical Society, 136(25), 8839–8842. https://doi.org/10.1021/ja5044787
Sknar, Y.E., Sknar, I.V., Savchuk, O.O., Danilov, F.I. (2020). Electrodeposition of Ni-Co alloy from methansulfonate electrolyte. The role of the electrolyte pH in the anomalous codeposition of nickel and cobalt. Surface and Coatings Technology, 387, 125542. https://doi.org/10.1016/j.surfcoat.2020.125542
Nenastina, T. A., Ved’, M. V., Proskurina, V. O., Zyubanova S. I. (2019). Electrochemical deposition of Co-Mo-W and Co-Mo-Zr coatings from complex electrolytes. Promising Materials and Processes in Applied Electrochemistry: monograph, Kyiv: KNUTD.
Belevskii, S. S., Bobanova, Zh. I., Buravets, V. A., Goteluak, A. V., Danil’chuck, V. V., Silkin, S. A., Dikusar A. I. (2016). Electrodeposition of Co-W coatings from boron gluconate electrolyte with a soluble tungsten anode. Russian Journal of Applied Chemistry, 89(9), 1427–1433. doi:10.1134/S107042721609007X
Tsyntsaru, N., Cesiulis, H., Budreika A. (2012). The effect of electrodeposition conditions and post-annealing on nanostructure of Co–W coatings. Surface and Coatings Technology, 206, 4262–4269. https://doi.org/10.1016/j.surfcoat.2012.04.036
Ćirović, N., Spasojević, P., Ribić-Zelenović, L., Mašković, P., Spasojević, M. (2015). Synthesis, Structure and Properties of Nickel-Iron-Tungsten Alloy Electrodeposits PART I: Effect of Synthesis Parameters on Chemical Composition, Microstructure and Morphology. Science of Sintering, 47, 347–365. doi: 10.2298/SOS1503347C
Karakurkchi, H. V. Ved’, M. V. Yermolenko, I. Iu. Sakhnenko, M. D. (2017). [Electrochemical coatings with iron alloys for surface hardening and protection]. Kharkiv: FOP Panov A. M. (In Ukrainian)
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

