SYNTHESIS, STRUCTURAL AND SPECTROSCOPIC CHARACTERIZATIONS, HIRSHFELD SURFACE ANALYSIS OF TWO COORDINATION COMPOUNDS ASSEMBLED FROM COPPER AND CARBOXYLATES, 3,5-DIMETHYL-1H-PYRAZOLE
DOI:
https://doi.org/10.15421/jchemtech.v31i3.276647Keywords:
pyrazole ligands, copper complexes, carboxylates, X-ray crystallography, Hirshfeld surface analysis, coordination polymerAbstract
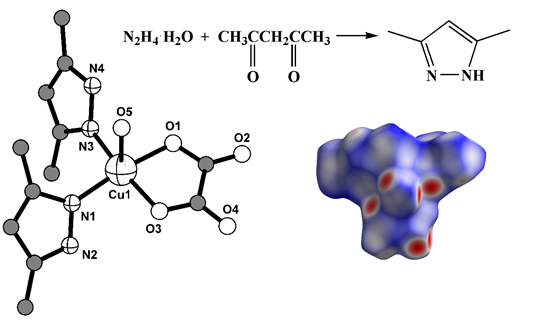
Two coordination compounds Cu(H2O)(DMPZ)2C2O4 (1) (DMPZ = 3,5-dimethyl-1H-pyrazole) and [Cu(H2O)2(CH3COO)Br]n (2) have been synthesized by oxidative dissolution method. The crystal structures of both compounds have been determined by single-crystal X-ray diffraction analysis. Complex 1 crystallizes in the triclinic P space group, while complex 2 is orthorhombic crystals in the space group Pnma. In both compounds, the Cu(II) ion displays a slightly distorted square-pyramidal coordination environment. In complex 1, the coordination number of copper(II) ion is five, with the equatorial position formed by two nitrogen atoms belonging to the two monodentate coordinated 3,5-dimethyl-1H-pyrazole molecules, and two O atoms of the oxalate anion coordinated in a chelate mode. The axial position is occupied by the O atom of the water molecule. In complex 2, the copper equatorial surrounding consists of four oxygen atoms from two trans-disposed water molecules and two acetate groups that are bridged in syn-anti mode with neighboring copper atoms. The apical coordination site is occupied by the bromide ion. Elemental analysis and spectroscopy characterization of the complexes and 3,5-dimethyl-1H-pyrazole are also reported. The Hirshfeld surface analysis suggests that the main contribution to intermolecular interactions in both compounds comes from hydrogen bonds (H···O/O···H and H···Br/Br···H) and other close contacts involving hydrogen atoms.
References
Viciano-Chumillas, M., Liu, X., Leyva-Pérez, A., Armentano, D., Ferrando-Soria, J., & Pardo, E. (2022). Mixed component metal-organic frameworks: Heterogeneity and complexity at the service of application performances. Coordination Chemistry Reviews, 451, 214273. https://doi.org/10.1016/j.ccr.2021.214273.
Feng, L., Wang, K. Y., Day, G. S., Zhou, H. C. (2019). The chemistry of multi-component and hierarchical framework compounds. Chemical Society Reviews, 48(18), 4823–4853. doi:10.1039/C9CS00250B
Begum, R., Rehman, M. U., Shahid, K., Haider, A., Iqbal, M., Tahir, M. N., & Ali, S. (2021). Synthesis, structural elucidation, DNA-binding and biological activity of nickel (II) mixed ligand carboxylate complexes. Journal of Molecular Structure, 1242, 130801. https://doi.org/10.1016/j.molstruc.2021.130801
Vasile Scăețeanu, G., Chifiriuc, M. C., Bleotu, C., Kamerzan, C., Măruţescu, L., Daniliuc, C. G., & Badea, M. (2018). Synthesis, structural characterization, antimicrobial activity, and in vitro biocompatibility of new unsaturated carboxylate complexes with 2, 2′-bipyridine. Molecules, 23(1), 157. https://doi.org/10.3390/molecules23010157
Das, B., Rahaman, A., Shatskiy, A., Verho, O., Karkas, M. D., Åkermark, B. (2021). The impact of ligand carboxylates on electrocatalyzed water oxidation. Accounts of Chemical Research, 54(17), 3326–3337. https://doi.org/10.1021/acs.accounts.1c00298
Pazoki, H., Anbia, M. (2019). Synthesis of a microporous copper carboxylate metal organic framework as a new high capacity methane adsorbent. Polyhedron, 171, 108–111. https://doi.org/10.1016/j.poly.2019.07.013
Zeng, M., Chen, X., & Kou, H. Z. (2021). Synthesis, Crystal Structure and Magnetic Properties of 1D Chain Complexes Based on Azo Carboxylate Oxime Ligand. Magnetochemistry, 7(7), 105. https://doi.org/10.3390/magnetochemistry7070105
Szymańska, I. B., Madajska, K., Butrymowicz, A., Barwiołek, M. (2021). Copper (II) Perfluorinated Carboxylate Complexes with Small Aliphatic Amines as Universal Precursors for Nanomaterial Fabrication. Materials, 14(23), 7451. https://doi.org/10.3390/ma14237451
Sidorov, A. A., Gogoleva, N. V., Bazhina, E. S., Nikolaevskii, S. A., Shmelev, M. A., Zorina-Tikhonova, E. N., Eremenko, I. L. (2020). Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates. Pure and Applied Chemistry, 92(7), 1093–1110. https://doi.org/10.1515/pac-2019-1212
Charalambous, M., Moushi, E. E., Nguyen, T. N., Papatriantafyllopoulou, C., Nastopoulos, V., Christou, G., & Tasiopoulos, A. J. (2019). Giant Heterometallic [Mn36Ni4] 0/2− and [Mn32Co8]“Loops-of-Loops-and-Supertetrahedra” Molecular Aggregates. Frontiers in Chemistry, 7, 96. https://doi.org/10.3389/fchem.2019.00096
Feng, X., Shang, Y., Zhang, H., Li, R., Wang, W., Zhang, D., Li, Z. (2019). Enhanced luminescence and tunable magnetic properties of lanthanide coordination polymers based on fluorine substitution and phenanthroline ligand. RSC advances, 9(29), 16328–16338. https://doi.org/10.1039/C9RA01574D
Kandel, Sh., Sathish, V., Mathivathanan, L., Morozov, A. N., Mebel, A. M., Raptis, R. G. (2019). Aggregation induced emission enhancement (AIEE) of tripodal pyrazole derivatives for sensing of nitroaromatics and vapor phase detection of picric acid. New J. Chem, 43, 7251–7258. https://doi.org/10.1039/C9NJ00166B
Fujisawa, K., Nemoto, T., Morishima, Y., Leznoff, D. B. (2021). Synthesis and Structural Characterization of a Silver(I) Pyrazolato Coordination Polymer . Molecules, 26, 1015. https://doi.org/10.3390/molecules26041015
Zhan, Sh.-Z., Chen, W., Lu, W., Zheng, J., Ding, F., Feng, T., Li, D. (2019). Counteranion-Triggered and Excitation-Dependent Chemopalette Effect in a Supramolecular Dual-Emissive System Based on Cu3Pz3. Inorg. Chem., 58, 1081–1090. https://doi.org/10.1021/acs.inorgchem.8b02203
Yu, F., Ji, B.-Q., Jagodic, M., Su, Y.-M., Zhang, Sh.-Sh., Feng, L., Kurmoo, M., Jaglicic, Z., Sun, D. (2020). Copper(II)-Assisted Ligand Fragmentation Leading to Three Families of Metallamacrocycle. Inorg. Chem., 59, 18, 13524–13532. https://doi.org/10.1021/acs.inorgchem.0c01915
Kreiger, D. I., Mathivathanan, L., Raptis, R. G. (2019). Coordination polymers based on pyrazole-4-carboxaldehyde-containing Cu3N6 metallacycles as building units. Cryst Eng Comm, 21, 3047–3055. https://doi.org/10.1039/C9CE00421A
Vynohradov, O. S., Pavlenko, V. A., Fritsky, I. O., Gural’skiy, I. A., Shova, S. (2020). Synthesis and Crystal Structure of Copper (II) 9-Azametallacrowns-3 with 4-Iodopyrazole. Russian Journal of Inorganic Chemistry, 65, 1481–1488. https://doi.org/10.1021/cr00019a006
Zaleski, C. M. (2022). Advances in Metallacrown Chemistry. Shippensburg, USA: Springer Nature.
Zhang, H. G., Du, Y. C., Yang, H., Zhuang, M. Y., Li, D. C., Dou, J. M. (2019). A new family of {Co4Ln8} metallacrowns with a butterfly-shaped structure. Inorganic Chemistry Frontiers, 6(7), 1904–1908. https://doi.org/10.1039/C9QI00661C
Pandolfo, L., Pettinari, C. (2017). Trinuclear copper (II) pyrazolate compounds: a long story of serendipitous discoveries and rational design. CrystEngComm, 19(13), 1701–1720. https://doi.org/10.1039/C7CE00009J
Elguero, J., Alkorta, I. (2020). A computational study of metallacycles formed by pyrazolate ligands and the coinage metals M= Cu (I), Ag (I) and Au (I):(pzM) n for n= 2, 3, 4, 5 and 6. Comparison with structures reported in the Cambridge Crystallographic Data Center (CCDC). Molecules, 25(21), 5108. https://doi.org/10.3390/molecules25215108
Davydenko, Y. M., Demeshko, S., Pavlenko, V. A., Dechert, S., Meyer, F., Fritsky, I. O. (2013). Synthesis, Crystal Structure, Spectroscopic and Magnetically Study of Two Copper(II) Complexes with Pyrazole Ligand. Zeitschrift Für Anorganische Und Allgemeine Chemie, 639(8-9), 1472–1476. https://doi.org/10.1002/zaac.201300078
Davydenko, Y. M., Diechert, S., Demeshko, S.O., Meyer, F., Pavlenko, V.A., Fritsky, I.O. (2013). Synthesis, structure and magnetic properties of copper(II) coordination polymer with 1H-pyrazole. Ukr. Khim. Zh., 79(6), 85–92. https://ucj.org.ua/index.php/journal/issue/view/49/6-2013
Tian, Y., Wang, Z. Y., Zang, S. Q., Li, D., Mak, T. C. (2019). Luminescent cyclic trinuclear coinage metal complexes with aggregation-induced emission (AIE) performance. Dalton Transactions, 48(7), 2275–2279. https://doi.org/10.1039/C8DT04898C
Mukherjee, R. (2000). Coordination chemistry with pyrazole-based chelating ligands: molecular structural aspects. Coordination Chemistry Reviews, 203(1), 151–218. https://doi.org/10.1016/S0010-8545(99)00144-7
Umakoshi, K., Yamauchi, Y., Nakamiya, K., Kojima, T., Yamasaki, M., Kawano, H., Onishi, M. (2003). Pyrazolato-bridged polynuclear palladium and platinum complexes. Synthesis, structure, and reactivity. Inorganic chemistry, 42(12), 3907–3916. https://doi.org/10.1021/ic026196g
Corrochano-Monsalve, M., González-Murua, C., Bozal-Leorri, A., Lezama, L., Artetxe, B. (2021). Mechanism of action of nitrification inhibitors based on dimethylpyrazole: A matter of chelation. Science of The Total Environment, 752, 141885. https://doi.org/10.1016/j.scitotenv.2020.141885
Deacon, G. B., Delbridge, E. E., Skelton, B. W., White, A. H. (1998). Unprecedented μ‐η2: η2‐Pyrazolate Coordination in [{Yb(η2‐tBu2pz)(μ‐η2:η2‐tBu2pz)(thf)}2]. Angewandte Chemie International Edition, 37(16), 2251–2252. https://doi.org/10.1002/(SICI)1521-3773(19980904)37:16%3C2251::AID-ANIE2251%3E3.0.CO;2-4
Deacon, G. B., Forsyth, C. M., Gitlits, A., Harika, R., Junk, P. C., Skelton, B. W., White, A. H. (2002). Pyrazolate Coordination Continues To Amaze – The New μ‐η2: η1 Binding Mode and the First Case of Unidentate Coordination to a Rare Earth Metal. Angewandte Chemie, 114(17), 3383–3385. https://doi.org/10.1002/1521-3757(20020902)114:17%3C3383::AID-ANGE3383%3E3.0.CO;2-0
Roy, M., Pal, A. K., Adhikary, A., Datta, A., Mondal, R. (2020). Paradoxical design of a serendipitous pyrazolate bridging mode: a pragmatic strategy for inducing ineluctable ferromagnetic coupling. Dalton Transactions, 49(39), 13704–13716. https://doi.org/10.1039/D0DT02468F
Pfeiffer, D., Heeg, M. J., & Winter, C. H. (2000). Synthesis and characterization of calcium complexes containing η2-pyrazolato ligands. Inorganic Chemistry, 39(11), 2377–2384. https://doi.org/10.1021/ic991049c
Perera, J. R., Heeg, M. J., Schlegel, H. B., & Winter, C. H. (1999). Ruthenium complexes bearing η5-pyrazolato ligands. Journal of the American Chemical Society, 121(18), 4536–4537. http://schlegelgroup.wayne.edu/Pub_folder/212.pdf
Cingolani, A., Galli, S., Masciocchi, N., Pandolfo, L., Pettinari, C., Sironi, A. (2006). The competition between acetate and pyrazolate in the formation of polynuclear Zn (II) coordination complexes. Dalton Transactions, 20, 2479–2486. https://doi.org/10.1039/B515630K
Sarma, R., Kalita, D., Baruah, J. B. (2009). Solvent induced reactivity of 3, 5-dimethylpyrazole towards zinc (II) carboxylates. Dalton Transactions. 36, 7428–7436. https://doi.org/10.1039/B905534G
Singh, U. P., Tyagi, P., Pal, S. (2009). Synthesis, structural and luminescence studies of some zinc complexes having pyrazole and carboxylate ligands. Inorganica Chimica Acta, 362(12), 4403–4408. https://doi.org/10.1016/j.ica.2009.06.018
Davydenko, Yu. M., Vitske, V. A., Pavlenko, V. A., Haukka, M., Vynohradov, O. S., Fritsky, I. O. (2022). Synthesis, crystal structure and properties of coordination polymers based on (3,5-dimethyl-1Н-pyrazole-4-yl)-acetic acid. Journal of Chemistry and Technologies, 30(2), 174–183. https://doi.org/10.15421/jchemtech.v30i2.252517
Davydenko, Yu. M., Fritsky, I.O., Pavlenko, V.O., Meyer F., Dechert S. (2009). Bis(acetato-κ2O,O’)bis(3,5-dimethyl-1H-pyrazole-κN2)copper(II). Acta Cryst., E65, m691–m692. https://doi.org/10.1107/S1600536809019400
Davydenko, Yu. M., Fritsky, I.O., Pavlenko, V.O., Meyer, F., Dechert, S. (2011). Chloridotris(3,5-dimethyl-1H-pyrazole-κN2)(formato-κO)copper(II)–dichloridobis (3,5-dimethyl-1H-pyrazole-κN2)copper(II) (2/1). Acta Cryst., E67, m732–m733. https://doi.org/10.1107/S1600536811016461
Davydenko, Y. M., Torre Fernandez, L., Roces Fernandez, L., Garcia-Granda, S., Pavlenko, V. O., Fritsky, I. O. (2011). Crystal structure and spectroscopic properties of the mononuclear copper(II) complex with 3,4,5-trimethyl-1Н-pyrazole ligand. Ukrainian Chemical Journal, 77(3-4), 7–10. https://ucj.org.ua/index.php/journal/issue/view/70/3-2018
Peng, C., Daizheng, L., Shiping, Y., Zonghui, J., Genglin, W., Xinkan, Y., Honggen, W. (1997). Crystal structure and ferromagnetic behavior of a μ-acetato-bridged one-dimensional linear-chain copper (II) complex. Inorganica chimica acta, 254(2), 371–373. https://doi.org/10.1016/S0020-1693(96)05162-6
Buvailo, A. I., Tomyn, S. V., Haukka, M., Pavlenko, V. A., Fritsky, I. O. (2008). Aquabis (3, 5-dimethyl-1H-pyrazole-κN)(oxalato-κ2O, O′) copper (II). Acta Crystallographica Section E: Structure Reports Online, 64(1), m37–m38. https://doi.org/10.1107/S1600536807058928
a) Sheldrick G. M., Acta Cryst. 2015, A71, 3-8; https://doi.org/10.1107/S2053273314026370
b) Sheldrick G. M., Acta Cryst. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218
Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., Verschoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper (II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1, 7-bis (N-methylbenzimidazol-2′-yl)-2, 6-dithiaheptane] copper (II) perchlorate. Journal of the Chemical Society, Dalton Transactions, (7), 1349–1356. https://doi:10.1039/dt9840001349.
Nakamoto, K. (2009). Infrared and Raman Spectra of Inorganic and Coordination Compounds. Hoboken, New Jersey: John Wiley&Sons.
Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D., Spackman, M. A. (2021). CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Journal of Applied Crystallo-graphy, 54(3), 1006–1011. https://doi.org/10.1107/S1600576721002910
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

