USING FILTER LOADING FOR IRON REMOVAL FROM WATER
DOI:
https://doi.org/10.15421/jchemtech.v31i2.277434Keywords:
iron, zeolite, filter, dirt capacity, adsorption film, water purificationAbstract
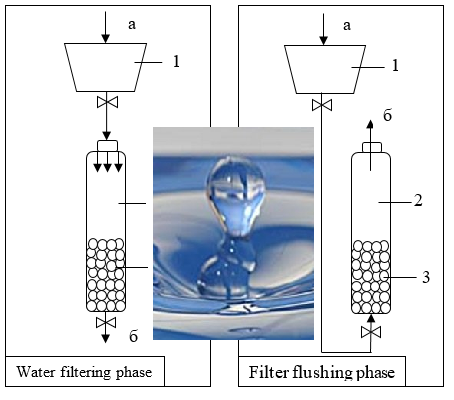
To purify water from iron compounds, were used granulated zeolite and zeolite modified with potassium permanganate. The experimental results showed that when filtering water, the modified zeolite removes iron from the aqueous environment better. The filtration cycle time depends on the initial concentrations of iron compounds in the water and continue until the critical level of resistance in the filtering unit is reached, due to the accumulation of sediment in the filter column. The calculation of the sedimentation rate on the surface of modified and unmodified zeolite has been carried out. The rate of formation of such a layer affects the efficiency of iron compound oxidation during water filtration. The rate of film formation on the loading surface in the case of filtration through the modified zeolite is greater, which indicates a more complete extraction of iron ions. After washing the filter, the modified zeolite did not lose its oxidizing power.
References
Remeshevska, I., Trokhymenko, G., Gurets, N., Stepova, O., Trus, I., Akhmedova, V. (2021). Study of the ways and methods of searching water leaks in water supply networks of the settlements of Ukraine. Ecological Engineering and Environmental Technology, 22(4), 14–21. https://doi.org/10.12912/27197050/137874
Trus, I., Radovenchyk, I., Halysh, V., Skiba, M., Vasylenko, I., Vorobyova, V., Sirenko, L. (2019). Innovative approach in creation of integrated technology of desalination of mineralized water. Journal of Ecological Engineering, 20(8), 107–113. doi:10.12911/22998993/110767
Trus, I., Gomelya, N., Halysh, V., Radovenchyk, I., Stepova, O., Levytska, O. (2020). Technology of the comprehensive desalination of wastewater from mines. Eastern-European Journal of Enterprise Technologies, 3(6-105), 21–27. https://doi.org/10.15587/1729-4061.2020.206443
Trus, I., Gomelya, M., Levytska, O., Pylypenko, T. (2022). Development of scaling reagent for waters of different mineralization. Ecological Engineering and Environmental Technology, 23(4), 81-87. doi:10.12912/27197050/150201
Trus, I., Gomelya M. (2021). Desalination of mineralized waters using reagent methods. Journal of Chemistry and Technologies, 29(3), 417–424. https://doi.org/10.15421/jchemtech.v29i3.214939
Trus, I., Gomelya, M. (2023). Applications of antiscalants in circulating water supply systems. Journal of Chemical Technology and Metallurgy, 58(2), 360–366.
Trus, I., Gomelya, M., Skiba, M., Pylypenko, T., Krysenko, T. (2022). Development of resource-saving technologies in the use of sedimentation inhibitors for reverse osmosis installations. Journal of Ecological Engineering, 23(1), 206–215. doi:10.12911/22998993/144075
Kalvani, N., Mesdaghinia, A., Yaghmaeian, K., Abolli, S., Saadi, S., Alimohammadi, M., Rashidi Mehrabadi, A. (2021). Evaluation of iron and manganese removal effectiveness by treatment plant modules based on water pollution index; a comprehensive approach. Journal of Environmental Health Science and Engineering, 19, 1005–1013. https://doi.org/10.1007/s40201-021-00665-2
Valentini, M. H. K., dos Santos, G. B., Franz, H. S., da Silva, L. A., da Silva Fraga, G., de Mello, N. P., ... & Romani, R. F. (2022). Analysis of the Influence of Climatic Factors on the Concentration of Iron and Manganese in Raw Water Intended for a Water Treatment System. Revista Brasileira de Geografia Física, 15(05), 2486–2499. https://periodicos.ufpe.br/revistas/rbgfe/article/viewFile/253965/41893
Usman, U. A., Yusoff, I., Raoov, M., Alias, Y., Hodgkinson, J., Abdullah, N., Hussin, N. H. (2021). Natural sources of iron and manganese in groundwater of the lower Kelantan River Basin, North-eastern coast of Peninsula Malaysia: water quality assessment and an adsorption-based method for remediation. Environmental Earth Sciences, 80(12), 425. https://doi.org/10.1007/s12665-021-09717-0
Martynov, S., Fylypchuk, V., Zoshchuk, V., Kunytskyi, S., Safonyk, A., Pinchuk, O. (2018). Technological model of water contact iron removal. Journal of Water and Land Development, (39), 93–99. doi: 10.2478/jwld-2018-0063
Martynov, S. Y., Poliakov, V. L. (2022). Experimental studies of iron transformations kinetics and autocatalysis during its physicochemical removal from underground water. Water Supply, 22(3), 2883–2895. https://doi.org/10.2166/ws.2021.428
Trus, I., Halysh, V., Gomelya, M., & Radovenchyk, V. (2021). Low-waste technology for water purification from iron ion. Ecological Engineering and Environmental Technology, 22(4), 116–123. doi: https://doi.org/10.12912/27197050/137860
Khatri, N., Tyagi, S., & Rawtani, D. (2017). Recent strategies for the removal of iron from water: A review. Journal of Water Process Engineering, 19, 291-304. https://doi.org/10.1016/j.jwpe.2017.08.015
Serrano, L. Z., Lara, N. O., Vera, R. R., Cholico-González, D. (2021). Removal of Fe (III), Cd (II), and Zn (II) as hydroxides by precipitation–flotation system. Sustainability, 13(21), 11913. https://doi.org/10.3390/su132111913
Trus, I., Gomelya, N., Trokhymenko, G., Magas, N., & Hlushko, O. (2019). Determining the influence of the medium reaction and the technique of magnetite modification on the effectiveness of heavy metals sorption. Eastern-European Journal of Enterprise Technologies, 6(10-102), 49–54. https://doi.org/10.15587/1729-4061.2019.188295
Trus, I., Gomelya, M., Chuprinov, E., Pylypenko, T. (2021). Optimization of dose calculation of modified magnetite during sorption purification of water from copper ions to create environmentally friendly technology, E3S Web of Conferences, 280, 10001. https://doi.org/10.1051/e3sconf/202128010001
Gomelya, M., Tverdokhlib, M., Shabliy, T., Linyucheva, O. (2021). Usage of sorbent-catalyst to accelerate the oxidation of manganese. Journal of Ecological Engineering, 22(4), 232–239. https://doi.org/10.12911/22998993/133350
Krupińska, I. (2019). Removal of iron and organic substances from groundwater in an alkaline medium. Journal of Environmental Engineering and Landscape Management, 27(1), 12–21. https://doi.org/10.3846/jeelm.2019.7726
Thinojah, T., Ketheesan, B., & Herath, G. B. B. (2020). Design of up-flow aerated filters for the removal of iron from groundwater. Water Supply, 20(8), 3233–3241. https://doi.org/10.2166/ws.2020.229
Yang, H., Tang, X., Luo, X., Li, G., Liang, H., Snyder, S. (2021). Oxidants-assisted sand filter to enhance the simultaneous removals of manganese, iron and ammonia from groundwater: formation of active MnOx and involved mechanisms. Journal of Hazardous Materials, 415, 125707. https://doi.org/10.1016/j.jhazmat.2021.125707
Das, D., Nandi, B. K. (2019). Removal of Fe (II) ions from drinking water using Electrocoagulation (EC) process: Parametric optimization and kinetic study. Journal of Environmental Chemical Engineering, 7(3), 103116. https://doi.org/10.1016/j.jece.2019.103116
Trus, I., Halysh, V., Radovenchyk, Y., & Fleisher, H. (2020). Conditioning of iron-containing solutions. Journal of Chemical Technology and Metallurgy, 55(2), 486–491.
Das, D., Nandi, B. K. (2020). Simultaneous removal of fluoride and Fe (II) ions from drinking water by electrocoagulation. Journal of Environmental Chemical Engineering, 8(1), 103643. https://doi.org/10.1016/j.jece.2019.103643
Tang, X., Wang, J., Zhang, H., Yu, M., Guo, Y., Li, G., Liang, H. (2021). Respective role of iron and manganese in direct ultrafiltration: from membrane fouling to flux improvements. Separation and Purification Technology, 259, 118174. https://doi.org/10.1016/j.seppur.2020.118174
Tang, X., Qiao, J., Wang, J., Huang, K., Guo, Y., Xu, D., Liang, H. (2021). Bio-cake layer based ultrafiltration in treating iron-and manganese-containing groundwater: Fast ripening and shock loading. Chemosphere, 268, 128842. https://doi.org/10.1016/j.chemosphere.2020.128842
Marsidi, N., Hasan, H. A., Abdullah, S. R. S. (2018). A review of biological aerated filters for iron and manganese ions removal in water treatment. Journal of Water Process Engineering, 23, 1–12. https://doi.org/10.1016/j.jwpe.2018.01.010
Marín-Rivera, J. V., Martínez-Girón, J., Quintero-Angel, M., Salcedo-Reyes, J. C. (2019). Effectiveness of vertical subsurface wetlands for iron and manganese removal from wastewater in drinking water treatment plants. Universitas Scientiarum, 24(1), 135–163. https://doi.org/10.11144/javeriana.sc24-1.eovs
Zeng, H., Yin, C., Zhang, J., Li, D. (2019). Start-up of a biofilter in a full-scale groundwater treatment plant for iron and manganese removal. International journal of environmental research and public health, 16(5), 698. https://doi.org/10.3390/ijerph16050698
Diaz-Alarcón, J. A., Alfonso-Pérez, M. P., Vergara-Gómez, I., Díaz-Lagos, M., Martínez-Ovalle, S. A. (2019). Removal of iron and manganese in groundwater through magnetotactic bacteria. Journal of environmental management, 249, 109381. https://doi.org/10.1016/j.jenvman.2019.109381
Trus, I., Radovenchyk, I., Halysh, V., Chuprinov, E., Benatov, D., Olena, H., Sirenko, L. (2022). Innovative method for water deiron ions using capillary material. Journal of Ecological Engineering, 23(3), 174–182. doi:10.12911/22998993/145467
Radovenchyk, I., Trus, I., Halysh, V., & Krysenko, T. (2022). Methods of processing liquid waste concentrates using materials with capillary properties. Journal of Chemical Technology and Metallurgy, 57(5), 946–952.
Trus, I., Radovenchyk, I., Halysh, V., Chuprinov, E., Benatov, D., Olena, H., Sirenko, L. (2022). Innovative method for water deiron ions using capillary material. Journal of Ecological Engineering, 23(3), 174–182. doi:10.12911/22998993/145467
Haldar, D., Duarah, P., Purkait, M. K. (2020). MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere, 251, 126388. https://doi.org/10.1016/j.chemosphere.2020.126388
Pandey, G., Tharmavaram, M., Phadke, G., Rawtani, D., Ranjan, M., Sooraj, K. P. (2022). Silanized halloysite nanotubes as ‘nano-platform’for the complexation and removal of Fe (II) and Fe (III) ions from aqueous environment. Separation and Purification Technology, 293, 121141. https://doi.org/10.1016/j.seppur.2022.121141
Pandey, G., Tharmavaram, M., Phadke, G., Rawtani, D., Ranjan, M., Sooraj, K. P. (2022). Silanized halloysite nanotubes as ‘nano-platform’for the complexation and removal of Fe (II) and Fe (III) ions from aqueous environment. Separation and Purification Technology, 293, 121141. https://doi.org/10.1016/j.seppur.2022.121141
Lazaratou, C. V., Panagiotaras, D., Panagopoulos, G., Pospíšil, M., Papoulis, D. (2020). Ca treated Palygorskite and Halloysite clay minerals for Ferrous Iron (Fe+2) removal from water systems. Environmental Technology & Innovation, 19, 100961. https://doi.org/10.1016/j.eti.2020.100961
Tang, C., Ramírez-Hernández, M., Thomas, B., Asefa, T. (2022). Selective and efficient extraction of iron from water systems with a recyclable phytate-polyaniline hydrogel. Journal of Cleaner Production, 380, 135006. https://doi.org/10.1016/j.jclepro.2022.135006
Zareh, M. M., El-Sayed, A. S., El-Hady, D. M. (2022). Biosorption removal of iron from water by Aspergillus niger. npj Clean Water, 5(1), 58. https://doi.org/10.1038/s41545-022-00201-1
Musah, B. I., Xu, Y., Liang, C., Peng, L. (2022). Biosorption of chromium (VI), iron (II), copper (II), and nickel (II) ions onto alkaline modified Chlorella vulgaris and Spirulina platensis in binary systems. Environmental Science and Pollution Research, 29(41), 62514–62536. https://doi.org/10.1007/s11356-022-19725-7
Hassouna, M. E. M., Marzouk, M. A., Elbably, M. A., El Maghrabi, A. H. (2018). Biosorption of iron by amended Aspergillus versicolor from polluted water sources. Biom. Biostat. Int. J, 7(6), 502–513. doi: 10.15406/bbij.2018.07.00253
Kim, H., Ko, R. A., Lee, S., Chon, K. (2020). Removal efficiencies of manganese and iron using pristine and phosphoric acid pre-treated biochars made from banana peels. Water, 12(4), 1173. https://doi.org/10.3390/w12041173
Nilavazhagi, A., Felixkala, T. (2021). Adsorptive removal of Fe (II) ions from water using carbon derived from thermal/chemical treatment of agricultural waste biomass: Application in groundwater contamination. Chemosphere, 282, 131060. https://doi.org/10.1016/j.chemosphere.2021.131060
Kang, H., Liu, Y., Li, D., Xu, L. (2022). Study on the Removal of Iron and Manganese from Groundwater Using Modified Manganese Sand Based on Response Surface Methodology. Applied Sciences, 12(22), 11798. https://doi.org/10.3390/app122211798
Bandar, S., Anbia, M., Salehi, S. (2021). Comparison of MnO2 modified and unmodified magnetic Fe3O4 nanoparticle adsorbents and their potential to remove iron and manganese from aqueous media. Journal of Alloys and Compounds, 851, 156822. https://doi.org/10.1016/j.jallcom.2020.156822
Trus, I., Gomelya, M., Chuprinov, E., Pylypenko, T. (2021). Optimization of dose calculation of modified magnetite during sorption purification of water from copper ions to create environmentally friendly technology. Paper presented at the E3S Web of Conferences, 280. doi:10.1051/e3sconf/202128010001
Trus, I., Gomelya, N., Trokhymenko, G., Magas, N., & Hlushko, O. (2019). Determining the influence of the medium reaction and the technique of magnetite modification on the effectiveness of heavy metals sorption. Eastern-European Journal of Enterprise Technologies, 6(10-102), 49–54. doi:10.15587/1729-4061.2019.188295
Chmielewská, E. (2019). Natural zeolite: Alternative adsorbent in purification or post-treatment of waters. In Modified clay and zeolite nanocomposite materials, 87–112. https://doi.org/10.1016/B978-0-12-814617-0.00012-8
Limaa, L. A., Silvab, Y. F., Limac, P. L. T. (2021). Iron removal efficiency in irrigation water by a zeolite added to sand media filters. Desalination and Water Treatment, 220, 241–245. doi: 10.5004/dwt.2021.27024
Krstić, V. (2021). Role of zeolite adsorbent in water treatment. In Handbook of Nanomaterials for Wastewater Treatment, 417–481. https://doi.org/10.1016/B978-0-12-821496-1.00024-6
De Souza, V.C.; Villarroel-Rocha, J.; De Araújo, M.J.G.; Sapag, K.; Pergher, S.B.C. (2018). Basic Treatment in Natural Clinoptilolite for Improvement of Physicochemical Properties. Minerals, 8, 595. https://doi.org/10.3390/min8120595
Huang, T., Yan, M., He, K., Huang, Z., Zeng, G., Chen, A., Chen, G. (2019). Efficient removal of methylene blue from aqueous solutions using magnetic graphene oxide modified zeolite. Journal of colloid and interface science, 543, 43–51. https://doi.org/10.1016/j.jcis.2019.02.030
Rad, L. R., Anbia, M. (2021). Zeolite-based composites for the adsorption of toxic matters from water: A review. Journal of Environmental Chemical Engineering, 9(5), 106088. https://doi.org/10.1016/j.jece.2021.106088
Chmielewská, E. (2019). Chapter 4 – Natural zeolite: Alternative adsorbent in purification or post-treatment of waters. In Micro and Nano Technologies, Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 87–112.
Poliakov, V., Martynov, S. (2021). Mathematical modeling of physicochemical iron removal from groundwater at rapid filters. Chemical Engineering Science, 231, 116318. https://doi.org/10.1016/j.ces.2020.116318
Martynov, S. Y., Poliakov, V. L. (2022). Experimental studies on the hydrodynamic properties of a deposit in rapid filters during physicochemical removal of iron from groundwater. Water Supply, 22(10), 7603–7617. https://doi.org/10.2166/ws.2022.305
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

