BICYCLIC METHYLENE AZIRIDINE IN REACTIONS WITH C- AND N-NUCLEOPHILES
DOI:
https://doi.org/10.15421/jchemtech.v31i3.278814Keywords:
azaheterocycles, 1,4-vinylation, vinylmagnesium bromide, aminolysisAbstract
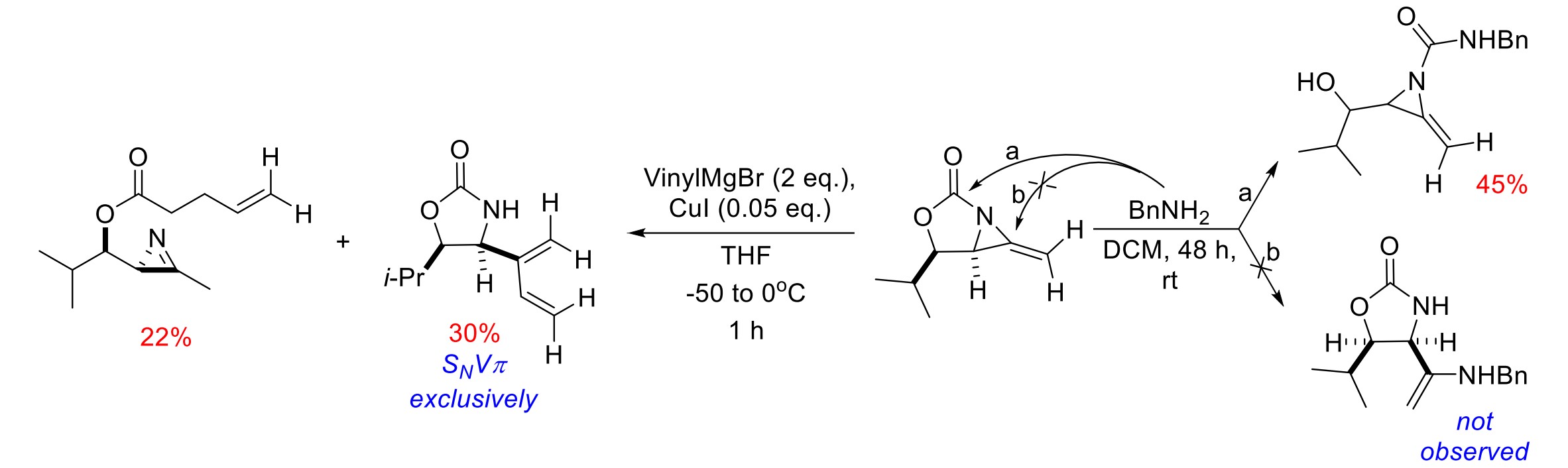
In recent years, various catalytic synthetic methods, starting with allenes, have been developed to produce a wide range of bicyclic methylene aziridines (MA). MA are valuable intermediates for the preparation of complex amine-containing stereotriads, tetrads, and heterocycles potentially useful in the synthesis of natural products and pharmaceuticals. This work reports the possibilities of using ring opening reactions of MA in the synthesis of biologically relevant heterocyclic motifs such as aziridineurea, 2H-azirine and 4,5-disubstituted oxazolidin-2-one. We performed detailed studies on the reaction between model MA with vinylmagnesium bromide and found that along with formal nucleophilic vinylic substitution product minor fraction of 2-methyl-1-(3-methyl-2H-azirin-2-yl)propyl pent-4-enoate was isolated. We showed that investigated reactions are stereoretentive, ruling out a SNVπ pathway, but the detailed mechanism remains open to speculation. Thus, reactivity patterns shown here for MA reactions can be useful in predicting reaction paths with other C- and N-nucleophiles. 2D NMR spectra of the key products were studied in detail including COSY, NOESY and HSQC experiments.
References
Gerstner, N. C., Adams, C. S., Tretbar, M., Schomaker, J. M. (2016). Stereocontrolled Syntheses of Seven-Membered Carbocycles by Tandem Allene Aziridination/[4+3] Reaction. Angew. Chem. Int. Ed., 55, 13240. https://doi.org/10.1002/anie.20160619
Burke, E. G., Schomaker, J. M. (2015). Oxidative Allene Amination for the Synthesis of Azetidin-3-ones. Angew. Chem. Int. Ed., 54, 12097. https://doi.org/10.1002/anie.20150472
Adams, C. S., Grigg, R. D., Schomaker, J. M. (2014). Aminosugar motifs via an allene aziridination strategy. Tetrahedron., 70, 4128. https://doi.org/10.1016/j.tet.2014.03.08
Rigoli, J. W., Weatherly, C. D., Vo, B. T., Neale, S., Meis, A. R., Schomaker, J. M. (2013). Chemoselective Allene Aziridination via Ag(I) Catalysis. Org. Lett., 15, 290. https://doi.org/10.1021/ol303167n
Rigoli, J. W., Weatherly, C. D., Alderson, J. M., Vo, B. T., Schomaker, J. M. (2013). Tunable, Chemoselective Amination via Silver Catalysis. J. Am. Chem. Soc., 135, 17238. https://doi.org/10.1021/ja406654y
Boralsky, L. A., Marston, D., Grigg, R. D., Hershberger, J. C., Schomaker, J. M. (2011). Allene Functionalization via Bicyclic Methylene Aziridines. Org. Lett., 13, 1924. https://doi.org/10.1021/ol2002418
Grigg, R. D., Schomaker, J. M., Timokhin, V. (2011). C–H amination/cyclocarbonylation of allene carbamates: a versatile platform for the synthesis of α,β-unsaturated γ-lactams. Tetrahedron., 67, 4318. https://doi.org/10.1016/j.tet.2011.03.02
Robertson, J., Feast, G. C., White, L. V., Steadman, V. A., Claridge, T. D. W. (2010). Structure and reactivity of bicyclic methylene aziridines prepared by intramolecular aziridination of allenes. Org. Biomol. Chem., 8, 3060. https://doi.org/10.1039/C003693E
Feast G. C., Page L. W., Robertson J. (2010). The intramolecular amination of allenes Chem. Commun., 46, 2835. https://doi.org/10.1039/B926179F
Liu, L., Ward, R. M., Schomaker, J. M. (2019). Mechanistic Aspects and Synthetic Applications of Radical Additions to Allenes. Chem. Rev., 119, 12422. https://doi.org/10.1021/acs.chemrev.9b00312
Mumford, P. M., Tarver, G. J., Shipman, M. (2009). Four-Component Reaction for the Preparation of α-Amino Phosphonates from Methyleneaziridines. J. Org. Chem., 74, 3573. https://doi.org/10.1021/jo9004958
Cariou, C. C. A., Clarkson, G. J., Shipman, M. (2008). Rapid Synthesis of 1,3,4,4-Tetrasubstituted β-Lactams from Methyleneaziridines Using a Four-Component Reaction. J. Org. Chem., 73, 9762. https://doi.org/10.1021/jo801664g
Shiers, J. J., Clarkson, G. J., Shipman, M., Hayes, J. F. (2006). Rapid generation of molecular complexity using “hybrid” multi-component reactions (MCRs): application to the synthesis of α-amino nitriles and 1,2-diamines. Chem. Commun., (6), 649. https://doi.org/10.1039/B516192D
Margathe, J.-F., Shipman, M., Smith, S. C. (2005). Solid-Phase, Multicomponent Reactions of Methyleneaziridines: Synthesis of 1,3-Disubstituted Propanones. Org. Lett., 7, 4987. https://doi.org/10.1021/ol051953a
Hayes, J. F., Shipman, M., Twin, H. (2002). Multicomponent Reactions Involving 2-Methyleneaziridines: Rapid Synthesis of 1,3-Disubstituted Propanones. J. Org. Chem., 67, 935. https://doi.org/10.1021/jo016164v
Hayes, J. F., Shipman, M., Twin, H. (2001). Asymmetric synthesis of 2-substituted piperidines using a multi-component coupling reaction: rapid assembly of (S)-coniine from (S)-1-(1-phenylethyl)-2-methyleneaziridine. Chem. Commun., (18), 1784. https://doi.org/10.1039/B106260N
Hayes, J. F., Shipman, M., Twin, H. (2000). Generation of metalloenamines by carbon–carbon bond formation: ring opening reactions of 2-methyleneaziridines with organometallic reagents. Chem. Commun., (18), 1791. https://doi.org/10.1039/B005623P
Prié, G., Prévost, N., Twin, H., Fernandes, S. A., Hayes, J. F., Shipman, M. (2004). A Lewis Acid Catalyzed Intramolecular [4+3] Cycloaddition Route to Polycyclic Systems That Contain a Seven-Membered Ring. Angew. Chem. Int. Ed., 43, 6517. https://doi.org/10.1002/anie.200461084
Quast, H., Vélez, C. A. W. (1974). 2-Lithiated 1-tert-Butyl-3-methyleneaziridine and Its Reaction Products. Angew. Chem. Int. Ed., 13, 342.
Palchykov, V., Dale, P. C., Robertson, J. (2021). Nucleophilic vinylic substitution in bicyclic methyleneaziridines: SNVπ or SNVσ? New J. Chem., 45, 9020. https://doi.org/10.1039/D1NJ01458G
Alper, H., Prickett, J. E. (1977). Metal carbonyl induced reactions of azirines. Coupling and insertion by diiron enneacarbonyl. Inorg. Chem., 16, 67. https://doi.org/10.1021/ic50167a016
Singh, G. S. (2016). Synthetic Aziridines in Medicinal Chemistry: A Mini-Review. Mini Rev. Med. Chem., 16, 892. https://doi.org/10.2174/1389557515666150709122244
Remers, W. A., Dorr, R. T. (2012). Chemistry and Pharmacology of Imexon and Related Cyanoaziridines. Curr. Med. Chem., 19, 5745. https://doi.org/10.2174/092986712803988802
Iyengar, B. S., Dorr, R. T., Alberts, D. S., Hersh, E. M., Salmon, S. E., Remers, W. A. (1999). Novel Antitumor 2-Cyanoaziridine-1-carboxamides. J. Med. Chem., 42, 510. https://doi.org/10.1021/jm980600x
Wells, G. M., Dudding, T., Belding, L., Frick, J. A., Nayek, A., Huang, J., Katz, S. J., Bergmeier, S. C. (2012). Studies on the ring opening reactions of 3-oxa-1-azabicyclo[3.1.0]hexan-2-ones. Synthesis of aminomethyl oxazolidinones and aziridinyl ureas. Tetrahedron., 68, 3980. https://doi.org/10.1016/j.tet.2012.03.071
Anupam, R., Nayek, A., Green, N. J., Grundy, F. J., Henkin, T. M., Means, J. A., Bergmeier, S. C., Hines, J. V. (2008). 4,5-Disubstituted oxazolidinones: High affinity molecular effectors of RNA function. Bioorg. Med. Chem. Lett., 18, 3541. https://doi.org/10.1016/j.bmcl.2008.05.015
Adams, C. S., Boralsky, L. A., Guzei, I. A., Schomaker, J. M. (2012). Modular Functionalization of Allenes to Aminated Stereotriads. J. Am. Chem. Soc., 134, 10807. https://doi.org/10.1021/ja304859w
Kas'yan, L. I., Pal'chikov, V. A., Bondarenko, Y. S. (2011). Azacycloalkanes from epoxides and aziridines. Russ. J. Org. Chem., 47, 1609. https://doi.org/10.1134/S1070428011110017
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

