MODELING OF THE ADSORPTIVE WATER REMOVAL FROM DICHLOROMETHANE USING THE ASPEN ADSORPTION PROGRAM: DESORPTION STAGE
DOI:
https://doi.org/10.15421/jchemtech.v31i2.279050Keywords:
solvent technology, dehydration process, adsorption, desorption, dichloromethane, zeolites, process modeling of chemical technology, Aspen Adsorption, design of processes and apparatus for chemical productionAbstract
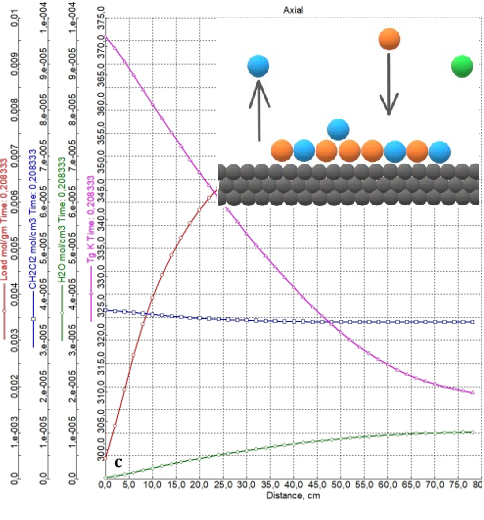
The purpose of this study is to investigate the water desorption stage in the technological process of dichloromethane dehydration and to provide recommendations on the choice of a desorption agent and optimal conditions for its use. The author performed computer modeling in the Aspen Adsorption program, which provided data for comparing the efficiency of using nitrogen and dichloromethane vapor as desorbing agents. The study showed that the use of dichloromethane vapor is characterized by significantly higher energy consumption than the use of nitrogen. This is primarily due to the large amount of energy required to vaporize dichloromethane before it is introduced into the column. In addition, the endothermic nature of the process causes the dichloromethane vapor to condense and form a liquid layer in the column, which increases the desorption time. Therefore, from a technological point of view, nitrogen is a more acceptable desorbing agent than dichloromethane, and desorption should be carried out at temperatures not lower than 80 °C.
References
Klei A., Moder, M., Stockdale O., Weihe U., Winkler, G. (2017). Digital in chemicals: From technology to impact https://www.mckinsey.com/industries/chemicals/our-insights/digital-in-chemicals-from-technology-to-impact#/.
Tangsriwong, K., Lapchit, P., Kittijungjit, T., Klamrassamee, T., Sukjai, Y., Laoonual, Y. (2020). Modeling of chemical processes using commercial and open source software: A comparison between Aspen Plus and DWSIM. IOP Conf. Series: Earth and Environmental Science 463, 012057 IOP Publishing https://doi.org/10.1088/1755-1315/463/1/012057.
Tian, Y., Demirel, S. E., Hasan, M. M. F., Pistikopoulos, E. N. (2018). An Overview of Process Systems Engineering Approaches for Process Intensification: State of the Art. Chem. Engineering and Processing - Process Intensification, 133, 160–210. https://doi.org/10.1016/j.cep.2018.07.014.
Towler, G., Sinnot, R. (2012). Chemical engineering design: Principles, Practice and Economics of Plant and Process Design, Elsevier Ltd. https://doi.org/10.1016/C2009-0-61216-2.
Jović, S., Laxminarayan, Ya., Keurentjes, J., Schouten, J., van der Schaaf, J. (2017). Adsorptive Water Removal from Dichloromethane and Vapor-Phase Regeneration of a Molecular Sieve 3A Packed Bed. Ind. Eng. Chem. Res., 56(17), 5042–5054. https://doi.org/10.1021/acs.iecr.7b00433.
Mekala, M., Neerudi, B., Are, P. R., Surakasi, R., Manikandan, G., Kakara, V. R., Abhaykumar, Dhumal, A. A. (2022) Water Removal from an Ethanol-Water Mixture at Azeotropic Condition by Adsorption Technique. Adsorption Science & Technology, 2022, 8374471. https://doi.org/10.1155/2022/8374471
Karimi, S., Yaraki, M., Karri, R. (2019). A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renewable and Sustainable Energy Reviews, 107, 535–553. https://doi.org/10.1016/j.rser.2019.03.025
Simo, M., Sivashanmugam, S., Brown, C.J., Hlavacek V. (2009). Adsorption/Desorption of Water and Ethanol on 3A Zeolite in Near-Adiabatic Fixed Bed. Ind. Eng. Chem. Res. 2009, 48, 9247–9260. https://pubs.acs.org/doi/10.1021/ie900446v.
van Kampen, J., Boon, J. & van Sint Annaland, M. (2021)/ Steam adsorption on molecular sieve 3A for sorption enhanced reaction processes. Adsorption. 2021, 27, 577–589. https://doi.org/10.1007/s10450-020-00283-8.
Поджарський М. А. (2022), Моделювання адсорбційного видалення води з дихлорметану з використанням програми Aspen Adsorption: стадія адсорбції. Journal of Chemistry and Technologies, 30(3), 441–450
Wood, K. R., Liu, Y. A., Yu, Y. (2018). Design, Simulation, and Optimization of Adsorptive and Chromatographic Separations: A Hands-On Approach, First Edition. Wiley-VCH Verlag GmbH & Co. KGaA.
ES288 Introduction to Aspen Adsorption. AspenTech Customer Education. Training Manual Course Number ES288.071.07. (2009). Aspen Technology, Inc.
Vuc̆elić, V., Dondur, V., Djurdjević, P., Vuc̆elić, D. (1976). An analysis of elementary processes of water desorption from zeolites of type a Part. I. Zeolites with monovalent counterions: undefined. Thermochimica Acta, 14(3), 341–347. https://doi.org/10.1016/0040-6031(76)85010-1.
Dondur, V., Vuc̆elić, V., Vuc̆elić, D., S̆us̆ić, M. (1976). An analysis of elementary processes of water desorption from zeolites of type a Part II. Zeolites with bivalent counterions. Thermochimica Acta, 14(3), 349–356. https://doi.org/10.1016/0040-6031(76)85011-3.
Palermo, A., Löffler, D. G. (1990). Kinetics of water desorption from pelletized 4A and 5A zeolites Thermochimica Acta, 159, 171–176. https://doi.org/10.1016/0040-6031(90)80105-8.
Planovsky, A. N., Ramm, V. M., Kagan, S. E. (1962). [Processes and apparatuses of chemical technology]. Moskow, USSR: Goskhimizdat (in Russian).
Timofeev D. P., Kabanova O. N. (1966). Kinetics of the desorption of water vapors from molded zeolites, types A and X. Bulletin of the Academy of Sciences of the USSR, Division of chemical science volume 15, 610–614.
Ruthven, D. M. (1984). Principles of adsorption and adsorption processes. New York, USA: John Wiley & Songs. https://www.scirp.org/(S(czeh2tfqw2orz553k1w0r45))/reference/referencespapers.aspx?referenceid=1295487.
Perez-Pellitero, J., Pirngruber, G. P. Industrial Zeolite Applications for Gas Adsorption and Separation Processes. In S. Valencia, F. Rey (Eds.) (2020), New Developments in Adsorption/Separation of Small Molecules by Zeolites. Springer Nature Switzerland AG. https://doi.org/10.1007/430_2020_75.
Saitake, M., Kubota, M., Watanabe, F., Matsuda H. (2007). Enhancement of Water Desorption from Zeolite by Microwave Irradiation. KAGAKU KOGAKU RONBUNSHU, 33(1), 53–58. https://doi.org/10.1252/kakoronbunshu.33.53.
Kubota, M., Hanada, T., Yabe, S., Kuchar, D., Matsuda H. (2011). Water desorption behavior of desiccant rotor under microwave irradiation. Applied Thermal Engineering, 31(8–9), 1482–1486. https://doi.org/10.1016/j.applthermaleng.2011.01.027.
Smejkal, T., Mikyška, J., Fučík R. (2020). Numerical modelling of adsorption and desorption of water vapor in zeolite 13X using a two-temperature model and mixed-hybrid finite element method numerical solver. International Journal of Heat and Mass Transfer, 148(2), https://doi.org/10.1016/j.ijheatmasstransfer.2019.119050.
Drying by adsorption. www.silica.de
Voc Treatment System Adsorption - Hot Nitrogen Desorption Type Vapor Recovery Unit. https://www.elevatedflaresystem.com/sale-10489238-voc-treatment-system-adsorption-hot-nitrogen-desorption-type-vapor-recovery-unit.html?from=wap.
Li, Y., Shen, Y., Niu, Z., Tian, Y., Zhang, D., Tang, Z., Li, W. (2023), Process analysis of temperature swing adsorption and temperature vacuum swing adsorption in VOCs recovery from activated carbon. Chinese Journal of Chemical Engineering, 53, 346–360. https://doi.org/10.1016/j.cjche.2022.01.029.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

