СHITOSAN FND CATIONIC STARCH AS A FLOCCULANTS FOR THE DISPERSION OF SILICON DIOXIDEСHITOSAN FND CATIONIC STARCH AS A FLOCCULANTS FOR THE DISPERSION OF SILICON DIOXIDE
DOI:
https://doi.org/10.15421/jchemtech.v31i3.279904Keywords:
cationizing reagent; cationic starch; chitosan; flocculation;. SiO2 dispersion; turbidimetry; thermal analysisAbstract
The polysaccharides chitosan with deacylation degree equal to 82% and cationic starch with a degree of hydrogen substitution in glucopyranose links for N,N,N-triethyl-N-2-hydroxypropylammonium fragment (20%) were used as flocculants in a 0.5% aqueous dispersion of ground quartz (silica, SiO2). Using the turbidimetric method it was shown that the flocculation rate of SiO2 particles depends on the pH value and the setting method of the dispersion medium pH.
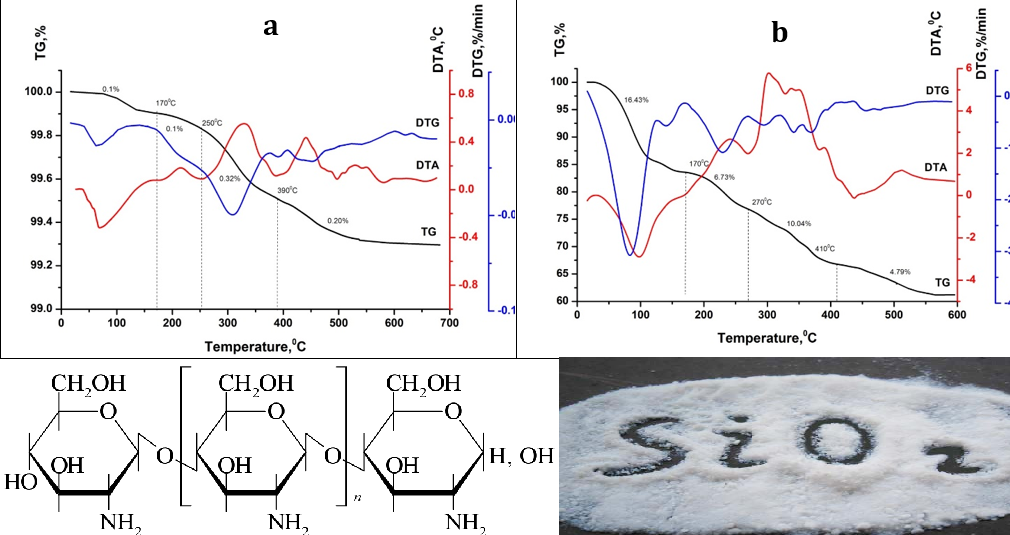
The turbidimetric method and thermal analysis show that effective flocculation occurs when the SiO2 dispersion is acidified to pH 2, followed by the addition of flocculant and setting the value of pH 5 by adding NaOH. This is explained by the greatest adsorption of cationic polysaccharides on the surface of SiO2 particles in the acidic environment (pH 2) of the SiO2 dispersion.
Flocculation in a 0.5% aqueous SiO2 dispersion has an extreme dependence on the concentration of flocculants, both chitosan and cationic starch. The highest flocculation rate is observed at a chitosan concentration of 588 mg/L and a cationic starch concentration of 560 mg/L. At lower concentrations the optimum density of adsorption layers on the dispersion particles surface is insufficient for effective flocculation, and at higher concentrations the adsorption layers of flocculants are formed, which partially stabilize the dispersed phase.
References
Vajihinejad, V., Gumfekar, S. P., Bazoubandi, B., Najafabadi,R. Z., Soares, J. B. P. (2018). Water Soluble Polymer Flocculants: Synthesis, Characterization, and Performance Assessment. Macromolecular Materials and Engineering, 304, 800526, 1‒43. https://doi.org/10.1002/mame.201800526
Cobbledick, J., Zhang, V., Rollings-Scattergood, S., Latulippe, D. R. (2017). Investigation of the role of flocculation conditions in recuperative thickening on dewatering performance and biogas production. Environmental Technology, 38(21), 2650–2660. https://doi:10.1080/09593330.2016.1272639
Su, Yu., Xu, Y., Xiong Sh., Zhao, S. (2015). Investigation in the flocculation performance of cationic starch flocculants. Academia Journal of Food Research, 3(1), 001–008. doi: 10.15413/ajfr.2015.0102
Sableviciene, D., Klimaviciute, R., Bendoraitiene, J., Zemaitaitis, A. (2005). Flocculation properties of high-substituted cationic starches. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 259(1-3), 23–30. https://doi.org/10.1016/j.colsurfa.2005.02.004
Ma, J., Fu, K., Fu, X., Guan, Q., Ding, L., Shi, J., Zhu, G., Zhang, X., Zhang, S., Jiang, L. (2017). Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Separation and Purification Technology, 182, 134‒143. https://doi.org/10.1016/j.seppur.2017.03.048
You, L., Lu, F., Li, D., Qiao, Z., Yin, Y. (2009). Preparation and flocculation properties of cationic starch/chitosan crosslinking-copolymer. Journal of Hazardous Materials. 172(1), 38‒45. https://doi.org/10.1016/j.jhazmat.2009.06.120
Salehizadeh, H., Yan, N., Farnood, R. (2018). Recent advances in polysaccharide bio-based flocculants. Biotechnology Advances. 36(1), 92–119. doi: 10.1016/j.biotechadv.2017.10.002
Li, H., Du, Yu., Wu, X., Zhan H. (2004). Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 242(1–3), 1–8. https://doi.org/10.1016/j.colsurfa.2004.04.051
Suresha, P.R., Manohar, V. Badiger (2022) Cationic chitosan graft flocculants: Synthesis, characterization and applications in kaolin separation. Materialstoday: Proseedings. https://doi.org/10.1016/j.matpr.2022.09.223
Elwakeel, Kh. Z., Atia Asem, A., Donia, A. M. (2009) Removal of Mo(VI) as oxoanions from aqueous solutions using chemically modified magnetic chitosan resins. Hydrometallurgy. 97(1-2), 21–28. https://doi.org/10.1016/j.hydromet.2008.12.009
Prado, H. J., Matulewicz, M. C. (2014) Cationization of polysaccharides: A path to greener derivatives with many industrial applications. European Polymer Journal. 52, 53–75. http://dx.doi.org/10.1016/j.eurpolymj.2013.12.011
Spinelli, V.A, Laranjeira, M.C.M., Favere, V.T. (2004) Preparation and characterization of quaternary chitosan salt: adsorption equilibrium of chromium(VI) ion. React Funct Polym. 61(3), 347–352. https://doi.org/10.1016/j.reactfunctpolym.2004.06.010,
Wang, X., Du, Y., Luo, J., Yang, J., Wang, W., Kennedy, J.F. (2009). A novel biopolymer/rectorite nanocomposite with antimicrobial activity. Carbohydr Polym. 77, 449–56. https://doi.org/10.1016/j.carbpol.2009.01.015
Popadyuk, N., Zholobko, O., Donchak, V., Harhay, K., Budishevska, O., Voronov, A., Kohut, A., Voronov, S. (2014). Ionically and Covalently Crosslinked Hydrogel Particles Based on Chitosan and Poly(ethylene glycol). Chemistry and chemical technology. (8)2, 171-176. http://nbuv.gov.ua/UJRN/Chemistry_2014_8_2_11
Budishevska, O., Popadyuk, N., Musyanovych, A., Kohut, A., Donchak, V., Voronov, A., Voronov, S. (2020). Formation of three-dimensional polymer structures through radical and ionic reactions of peroxychitosan. Studies in Natural Products Chemistry (Bioactive Natural Products). 64, 365–390. https://doi.org/10.1016/B978-0-12-817903-1.00012-7
Klimaviciute, R., Sableviciene, D., Bendoraitienė, J., Zemaitaitis, A. (2010) Kaolin dispersion destabilization with microparticles of cationic starches. Desalination and Water Treatment., 20, 243–252. https://doi.org/10.5004/dwt.2010.1547
Krentz, D.-O., Lohmann, C., Schwarz, S., Bratskaya, S., Liebert, T., Laube, J., Heinze, T., Kulicke W.-M. (2006). Properties and Flocculation Efficiency of Highly Cationized Starch Derivatives. Starch/Stдrke. 58(3-4), 161–169. https://doi.org/10.1002/star.200500431
Lekniute-Kyzike, E., Bendoraitiene, J., Danilovas, P. P., Algirdas, Z. (2016). A novel way to obtain effective cationic starch flocculants. Desalination and Water Treatment., 1–11.
https://doi.org/10.1080/19443994.2016.1138892
Kostyk, O. A., Budishevska, O.H., Vostres, V. B., Nadashkevych, Z. Y., Voronov, S. A. (2020). Cationic starches as flocculants. Journal of Chemistry and Technologies. 28(1), 17–26. https://doi.org/10.15421/082003
Yee T. Y., and Fatehah, M. O. (2017). Characterization and Transformation of Silicon Dioxide Nanoparticles in Aqueous Suspensions: Influence of pH. Iranian Journal of Energy and Environment, 8(4), 262-268. doi.10.5829/IJEE.2017.08.04.03
Moore, M.N.. (2006). Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International. (32), 967–976. https://doi.org/10.1016/j.envint.2006.06.014
Kostyk, O. A., Budishevska, O. H., Vostres, V. B., Nadashkevych, Z. Y., Voronov S. A. (2019). Cationation of starch with an aminating reagent based on triethylamine and epichlorohydrin. Voprosy khimii i khimicheskoi tekhnologii. (6), 113-120.
http://dx.doi.org/10.32434/0321-4095-2019-127-6-113-120
Chen, P., Xie, F., Zhao, L., Qiao, Q., Liu, X. (2017). Effect of acid hydrolysis on the multi-scale structure change of starch with different amylose content. Food Hydrocolloids, (69), 359–368. doi:10.1016/j.foodhyd.2017.03.003
Cheronis, T. S., Nicholas D., Ronzio, A. R., Ma. (1954). Micro and Semimicro Methods (Technique of Organic Chemistry, Volume VI). Interscience Publishers Inc.
Krasinskyi, V. V., Kochubei, V. V., Klym Y., Suberlyak, O. V. (2017). Thermogravimetric research into composites based on the mixtures of polypropylene and modified polyamide. Eastern-European Journal of Enterprise Technologies. 4/12(88), 44–49. https://doi.org/10.15587/1729-4061.2017.108465
Kokot, G., Bespalova, M. I., Krishnan M. (2016) Measured electrical charge of SiO2 in polar and nonpolar media. J. Chem. Phys. 145(19), 194701. https://doi.org/10.1063/1.4967401
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

