METHODS OF SYNTHESIS OF QUINAZOLINES AND THEIR CONDENSED ANALOGUES – POTENTIAL ANTI-INFLAMMATORY AGENTS (REVIEW)
DOI:
https://doi.org/10.15421/jchemtech.v31i2.280199Keywords:
quinazolones-4, quinazolin-4-amines, condensed and spiro-quinazolines, synthesis, anti-inflammatory activityAbstract
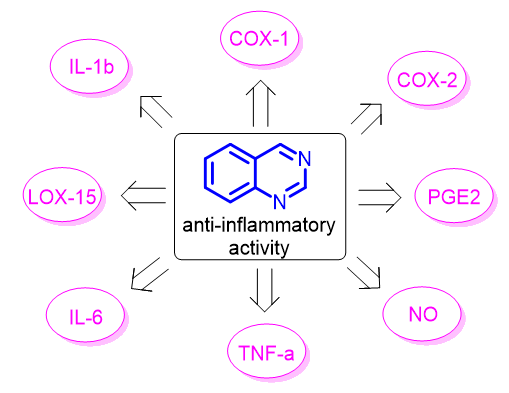
Present review is critical analysis of recently published data devoted to the search of the novel anti-inflammatory agents among substituted and condensed quinazolines. It has been shown that anti-inflammatory activity is typical for various quinazoline-containing compounds including substituted quinazolin-4-ones, quinazolin-4-amines, condensed and spiro-condensed quinazolines. The features of target compounds synthesis were systematized and discussed. It was shown that synthesis of quinazoline-containing anti-inflammatory agents in most of cases based on modification of anthranilic acid derivatives, substituted 2-aminobenzonitriles or isatoic anhydride. Particular attention was paid to the discussion of 2-azaheterylanilines as original initial compounds for synthesis of condensed quinazolines with promising anti-inflammatory activity. The mechanisms of quinazolines biological activity have been presented and discussed as well. It has been shown that described compounds can effect on COX-1, COX-2, LOX-15, NO, PGE2, IL-1b, IL-6, TNF-a and other typical for anti-inflammatory agents’ biomolecular targets. For some series of compounds, the structure-biological activity relationships were discussed. The data presented in review substantiates the reasonability of the search of new highly effective and low-toxic drugs with anti-inflammatory activity among quinazoline containing compounds.
References
Armarego, W.L.F., Brown, D.J., (ed). (1967). The Chemistry of Heterocyclic Compounds, Fused Pyrimidines (Part I; Vol. 24(1)), Interscience Publishers: N.-Y. - London - Sydney.
Brown, D.J., Suppl, I., Taylor, E.C., (ed). (1996). The Chemistry of Heterocyclic Compounds, (Vol. 55), J. Wiley & Sons Inc.: N.-Y. - Chichester - Brisbane - Toronto - Singapore - Weinheim.
Witt, A., Bergman, J. (2003). Recent Developments in the Field of Quinazoline Chemistry. Curr. Org. Chem, 7: 659–677. http://dx.doi.org/10.2174/1385272033486738
Connolly, D. J., Cusack, D., O’Sullivan, T. P., Guiry, P. J. (2005). Synthesis of quinazolinones and quinazolines. Tetrahedron, 61(43), 10153–10202. http://dx.doi.org/10.1016/j.tet.2005.07.010.
Wang, D., Gao, F. (2013). Quinazoline derivatives: synthesis and bioactivities. Chemistry Central Journal, 7(1), 95. http://dx.doi.org/10.1186/1752-153x-7-95;
Vinod G. Ugale, Sanjay B. Bari (2014). Quinazolines: New horizons in anticonvulsant therapy. Eur. J. Med. Chem., 80. 447–501. http://dx.doi.org/10.1016/j.ejmech.2014.04.072.
Mohammad, A. (2014). Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. International J. Med. Chem, 27, 1–27. http://dx.doi.org/10.1155/2014/395637.
Dumitrascu, F., Popa, M. M. (2014). Pyrrolo[1,2-a]–quinazolines. Synthesis and biological properties. ARKIVOC., (i). 428–452. http://dx.doi.org/10.3998/ark.5550190.p008.699.
He, L., Li, H., Chen, J., Wu, X.-F. (2014). Recent advances in 4(3H)-quinazolinone syntheses. RSC Adv., 4(24): 12065–12077. http://dx.doi.org/10.1039/c4ra00351a
Al-Ebaisat, H. S. (2015). A Review on Synthesis and Spectral Properties of Quinazolines and Pyrimidines. American Chemical Science Journal. 6(4), 213–223. http://dx.doi.org/10.9734/ACSj/2015/16128.
Chaban, Z., Harkov, S., Chaban, T., Klenina, O., Ogurtsov, V., Chaban, I. (2017). Recent advances in synthesis and biological activity evaluation of condensed thiazoloquinazolines: a review. Pharmacia. 64(3). 52–66.
Rakesh, K. P., Darshini, N., Shubhavathi, T., Mallesha, N. (2017). Biological Applications of Quinazolinone Analogues: A Review. Organic & Medicinal Chem IJ. 2(2), 555585. http://dx.doi.org/10.19080/OMCIJ.2017.02.555585.
Mohammadkhani, L., Heravi, M. M. (2020). Microwave-Assisted Synthesis of Quinazolines and Quinazolinones: An Overview. Frontiers in Chemistry, 8. http://dx.doi.org/10.3389/fchem.2020.580086
D'yakonov, A. L., Telezhenetskaya, M. V. (1997). Quinazoline alkaloids in nature. Chemistry of Natural Compounds, 33: 221–267;
Michael, J. P. (2001). Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 18: 543–550.
Mhaske, S. B. Argade, N. P. (2006). The Chemistry of Recently Isolated Naturally Occurring Quinazolinone Alkaloids. Tetrahedron, 62, 9787–9826. http://dx.doi.org/10.1016/j.tet.2006.07.098
Joseph, M. P. (2007). Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 24. 223–246.
Kshirsagar, U. A. (2015). Recent developments in the chemistry of quinazolinone alkaloids. Organic & Biomolecular Chemistry, 13(36), 9336–9352. http://dx.doi.org/10.1039/c5ob01379h.
Chandrika, P. M., Rao, R.R., Narsaiah, B., Raju, M.B. (2008). Quinazoline derivatives with potent anti-inflammatory and anti-allergic activities. Int. J. Chem. Sci., 6(3). 1119–1146.
Selvam, T. P., Kumar, P. V. (2011). Quinazoline Marketed drugs – A Review. Research in Pharmacy, 1(1). 1–21.
Parvez, A. N., Rana, А., Imran, М. (2011). An Updated Review: Newer Quinazoline Derivatives Under Clinical Trial. International J. Pharmaceutical & Biological Archives, 2(6), 1651–1657.
Marzaro, G., Guiotto, A., Chilin, A. (2012). Quinazoline derivatives as potential anticancer agents: a patent review (2007 - 2010). Expert Opin. Ther. Patents, 22(3), 223–252. http://dx.doi.org/10.1517/13543776.2012.665876.
Ravez, S., Castillo-Aguilera, O., Depreux, P., Goossens, L. (2015). Quinazoline derivatives as anticancer drugs: a patent review (2011 - present). Expert Opin. Ther. Patents, 25(7), 1–16. http://dx.doi.org/10.1517/13543776.2015.1039512
Alam, J., Alam, О., Naim, M.J., Alam, P. (2015). A review: Recent investigations on Quinazoline Scaffold. International J. Advanced Research. 3(12), 1656–1664.
Sukriti, S. (2015). Biological activity of Quinazoline: A Review. International Journal of Pharma Sciences and Research (IJPSR). 6(9), 1206–1213.
Jafari, E., Khajouei, M. R., Hassanzadeh, F., Hakimelahi, G. H., Khodarahmi, G. A. (2016). Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci., 11(1). 1–14.
Ismail, R. S. M., Ismail, N. S. M., Abuserii, S., Abou El Ella, D. A. (2016). Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future Journal of Pharmaceutical Sciences, 2(1), 9–19. http://dx.doi.org/10.1016/j.fjps.2016.02.001
Darwish, K.M., Dakhil, O.O. (2017). A Review on synthesis and biological profiles of some Quinazolines and (4H)-3,1-Quinazolin-4-ones of active substituents and their uses as starting materials in reaction schemes. Libyan Journal of Science & Technology, 6(1). 8–13.
Ajani, O.O., Audu, O. Y., Aderohunmu, D. V., Owolabi, F.E., Olomieja, A. O. (2017). Undeniable Pharmacological Potentials of Quinazoline Motifs in Therapeutic Medicine. American Journal of Drug Discovery and Development, 7: 1–24. http://dx.doi.org/10.3923/ajdd.2017.1.24.
Reddy, A. G., Babu, V. Ha., Prakash Rao, Y. J. (2017). A Review on Quinazolines as Anticancer Agents. Journal of Chemical and Pharmaceutical Sciences, 10(3): 1492–1504.
Shagufta, Ahmad, I. (2017). An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Med. Chem. Commun., 5, http://dx.doi.org/10.1039/c7md00097a.
Hameed, A., al-Rashida, M., Uroos, M., Ali, S.A., Ishtiaq, A.M., Khan, K.M. (2018). Quinazoline and Quinazolinone as Important Medicinal Scaffoldes: A Comparative Patent Review (2011-2016). Expert Opin. Ther. Patents, 28(4), 281–297 http://dx.doi.org/10.1080/13543776.2018. 1432596.
Auti P. S., George G., Paul A. T. (2020). Recent advances in the pharmacological diversification of quinazoline /quinazolinone hybrids. RSC Advances, 10(68), 41353–41392. http://dx.doi.org/10.1039/d0ra06642g.
Francis, J.E., Cash, W.D., Barbaz, W.D., Bernard, P.S., Lovell, R.A., Mazzenga G.C. (1991), Synthesis and benzodiazepine binding activity of a series of novel [1,2,4]triazolo[1,5-c]quinazolin-5(6H)-ones. J. Med. Chem. 34, 281–290. http://dx.doi.org/10.1021/jm00105a044.
Jantova, S., Cipak, L., Slamenova, D., Horvatha, V., Rauko, P. (2003). Induction of cytotoxicity and sDNA breaks by 9-bromo-5-morpholino-tetrazolo[1,5-c]quinazoline in tumor cells cultured in vitro. Toxicol. in Vitro. 17, 457–463. http://dx.doi.org/10.1016/S0887-2333(03)00066-3.
Okamura, T., Kurogi, Y., Hashimoto, K., Nishikawaa, H., Nagao, Y. (2004). Facile synthesis of fused 1,2,4-triazolo[1,5-c]pyrimidine derivatives as human adenosine A3 receptor ligands. Bioorg. Med. Chem. Lett., 14(10), 2443–2446. http://dx.doi.org/10.1016/j.bmcl.2004.03.010.
Kim, Y.-C., Xiao-duo, Ji., Jacobson, K. A. (1996). Derivatives of the Triazoloquinazoline Adenosine Antagonist (CGS15943) Are Selective for the Human A3 Receptor Subtype. J. Med. Chem, 39(21), 4142–4148. http://dx.doi.org/10.1021/JM960482I.
Kim Y.C., Zwart M.De., Chang L., Moro S., Kuenzel J., Melman N. (1998). Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) having high potency at the human A2B and A3 receptor subtypes. at al. J. Med. Chem., 41, 2835–2845. http://dx.doi.org/10.1021/jm980094b.
Gineinah, M.M., Nasr, M.N., Abdelal, A.M., El-Emam, A.A., Said, S.A. (2000). Synthesis and Antiinflammatory Evaluation of New 2- and 3-Substituted 1,2,4-Triazolo[4,3-c]- and [1,5-c]quinazoline Derivatives. Med. Chem. Res. 10(4), 243–252.
Balo, C., Lopes, C., Brea, J.M., Fernandez, F., Caamano, O. (2007). Synthesis and Evaluation of Adenosine Antagonist Activity of a Series of [1,2,4]Triazolo[1,5-c]quinazolines. Chem. Pharm. Bull., 55(3), 372–375 http://dx.doi.org/10.1248/cpb.55.372.
Avendano, C., Menendez, J. C. (2008). Medical chemistry of anticancer drugs. UK: Elsevier’s Science & Technology Rights Department inOxford.
Mani Chandrika, P., Raghu, A., Ram Rao, B., Narsaiah, M., Bhagawan Raju (2008). Quinazoline dersvatives with potent anti-inflammatory and antiallergic activities. Int. J. Chem. Sci., 6(3), 1119–1146.
Hussein, M. A. (2010). Structure anti-inflammatory activity relationship and biochemical evaluation of some novel triazoloquinazoline and triazino-quinazoline derivatives containing sulfacetamide moiety. Int. J. Appl. Biol. Pharm. Tech., 1(3), 1054–1066.
Kehler, J., Ritzen, A., Langgard, M., Petersen, S.L., Farah, M.M., Bundgaard, C. Christoffersen C. T., Nielsen J., Kilburn J. P. (2011). Triazoloquinazolines as a novel class of phosphodiesterase 10A (PDE10A) inhibitors. Bioorg. Med. Chem. Lett., 21, 3738–3742. http://dx.doi.org/10.1016/j.bmcl.2011.04.067
Levin, J. I., Laufer, S. (Eds) (2012). Anti-Inflammatory Drug Discovery. RSC Drug Discovery Series No. 26, Cambridge: Royal Society of Chemistry.
Khan, I., Ibrar, A., Аbbas, А., Abbas, N., Saeed, A. (2014). Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthesis approaches and multifarious applications. Eur. J. Med. Chem., 76, 193–244; htpp://dx.doi.org/10.1016/j.ejmech.2014.02.005.
Khan, I., Ibrar, A., Ahmed, W., Saeed, A. (2015). Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur. J. Med. Chem. 90, 124–169. http://dx.doi.org/10.1016/j.ejmech.2014.10.084.
Faisal, M., Saeed, A. (2021). Chemical Insights Into the Synthetic Chemistry of Quinazolines: Recent Advances. Front. Chem., https://doi.org/10.3389/fchem.2020.594717.
Ali Gamal Al-kaf (Ed.) (2020). Quinazolinone and Quinazoline Derivatives. Acad. Sana'a University. https://doi.org/10.5772/intechopen.85315.
Narendra, B. A., Rama, R. N. (2011). Synthesis of some new quinazolinone formazans as anti-Inflammatory and anthelmintic agents. Journal of Pharmacy Research, 4(4), 983–985.
Zayed, F. M., Hassan, H. M. (2014). Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharmaceutical Journal, 22(2). 157–162.
Kandpal, B., Meshram, J., Mohanram, I., Shaikh, A. (2014). Evaluation of newly synthesized quinazolinone derivatives of hydrazones as potent anti-inflammatory and antbacterial agents. Medicinal Chemistry Research, 24(4), 1419–1426. http://dx.doi.org/10.1007/s00044-014-1226-3
Ghodge, B., Kshirsagar, A., Navghare, V. (2020). Synthesis, characterization, and investigation of the anti-inflammatory effect of 2,3-disubstituted quinazoline-4(1H)-one. Beni-Suef University Journal of Basic and Applied Sciences. 9(1), 30 (2020). https://doi.org/10.1186/s43088-020-00056-w
El-Hashash, M. A. E.-A., Azab, M. E., Faty, R. A. E.-A., Amr, A. E.-G. E. (2016). Synthesis, Antimicrobial and Anti-inflammatory Activity of Some New Benzoxazinone and Quinazolinone Candidates. Chemical & Pharmaceutical Bulletin, 64(3), 263–271. http://dx.doi.org/10.1248/cpb.c15-00904.
Krishnarth, N., Verma, S. K., Chaudhary, A. (2020). Synthesis and Anti-Inflammatory Activity of Some Novel Quinazolinone Derivatives. FABAD J. Pharm. Sci., 45(3), 205–210.
Serya, R. A. T., Abbas, A. H., Ismail, N. S. M., Esmat, A., Abou El Ella, D. A. (2015). Design, Synthesis and Biological Evaluation of Novel Quinazoline-Based Anti-inflammatory Agents Acting as PDE4B Inhibitors. Chemical & Pharmaceutical Bulletin, 63(2). 102–116. https://doi.org/10.1248/cpb.c14-00737.
El-Feky, A., Imran, S., Nayeem, N. (2017). Design, Synthesis, and Anti-inflammatory Activity of Novel Quinazolines. Oriental Journal of Chemistry, 33(2), 707–716. https://doi.org/10.13005/ojc/330217
Mohamed, M.S., Kamel, M.M., Kassem, E.M., Abotaleb, N., Khedr, M., Ahmed, M.F. (2011). Synthesis, biological evaluation and molecular docking of quinazoline-4(1H)-one derivatives as anti-inflammatory and analgesic agents. Acta Pol Pharm., 68(5), 665–75.
Dash, B., Dash, S., Laloo, D., Medhi, C. (2017). Design, Synthesis and Preliminary Pharmacological Screening (antimicrobial, analgesic and anti-inflammatory activity) of Some Novel Quinazoline Derivatives. Journal of Applied Pharmaceutical Science, 7(06), 83–96. https://doi.org/10.7324/JAPS.2017.70612
Rajput, C.S., Singhal, S. (2013). Synthesis, Characterization, and Anti-Inflammatory Activity of Newer Quinazolinone Analogs. Journal of Pharmaceutics, 2013, 1–7. https://doi.org/10.1155/2013/907525
Sheorey, R., Thangathiruppathy, A., Alagarsamy, V. (2013). Synthesis, Analgesic and Anti-inflammatory Activities of 3- Ethyl-2-substituted Amino-3H-quinazolin-4-ones. Tropical Journal of Pharmaceutical Research, 12(4), 583–589. https://doi.org/10.4314/tjpr.v12i4.21
Abuelizz, H. A., Hassane, A. E., Marzouk, M., Ezzeldin, E., Ali, A. A., Al-Salahi, R. (2017). Molecular modeling, enzyme activity, anti-inflammatory and antiarthritic activities of newly synthesized quinazoline derivatives. Future Medicinal Chemistry, 9(17), 1995–2009. https://doi.org/10.4155/fmc-2017-0157
Alagarsamy, V., Solomon, V. R., Sulthana, M. T., Vijay, M.S., Narendhar, B. (2015). Design and synthesis of quinazolinyl acetamides for their analgesic and anti-inflammatory activities. Zeitschrift Für Naturforschung B, 70(8), 597–604. https://doi.org/10.1515/znb-2015-0035.
Farag, A. A., Khalifa, E. M., Sadik, N. A., Abbas, S. Y., Al-Sehemi, A. G., Ammar, Y. A. (2012). Synthesis, characterization, and evaluation of some novel 4(3H)-quinazolinone derivatives as anti-inflammatory and analgesic agents. Medicinal Chemistry Research, 22(1), 440–452. https://doi.org/10.1007/s00044-012-0046-6
Farag, D. B., Farag, N. A., Esmat, A., Abuelezz, S. A., Abdel-Salam, I. E., Abou El Ella, D. A. (2015). Synthesis, 3D pharmacophore, QSAR and docking studies of novel quinazoline derivatives with nitric oxide release moiety as preferential COX-2 inhibitors. MedChemComm, 6(2), 283–299. https://doi.org/10.1039/c4md00392f
Rakesh, K. P., Manukumar, H. M., Gowda, D. C. (2015). Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorganic & Medicinal Chemistry Letters, 25(5), 1072–1077. https://doi.org/10.1016/j.bmcl.2015.01.010
Abbas, S. E., Awadallah, F. M., Ibrahin, N. A., Said, E. G., Kamel, G. M. (2012). New quinazolinone–pyrimidine hybrids: Synthesis, anti-inflammatory, and ulcerogenicity studies. European Journal of Medicinal Chemistry, 53, 141–149. https://doi.org/10.1016/j.ejmech.2012.03.050
Abdel-Aziz, A. A.-M., Abou-Zeid, L. A., El Tahir, K. E. H., Ayyad, R. R., El-Sayed, M. A.-A., El-Azab, A. S. (2016). Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. European Journal of Medicinal Chemistry, 121, 410–421. https://doi.org/10.1016/j.ejmech.2016.05.066
Alafeefy, A. M., Kadi, A. A., Al-Deeb, O. A., El-Tahir, K. E. H., Al-jaber Nabila, A. (2010). Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. European Journal of Medicinal Chemistry, 45(11), 4947–4952. https://doi.org/10.1016/j.ejmech.2010.07.067
Hu, J., Zhang, Y., Dong, L., Wang, Z., Chen, L., Liang, D., Liang, G. (2014). Design, Synthesis, and Biological Evaluation of Novel Quinazoline Derivatives as Anti-inflammatory Agents against Lipopolysaccharide-induced Acute Lung Injury in Rats. Chemical Biology & Drug Design, 85(6), 672–684. https://doi.org/10.1111/cbdd.12454
Dvorakova, M., Langhansova, L., Temml, V., Pavicic, A., Vanek, T., Landa, P. (2021). Synthesis, Inhibitory Activity, and In Silico Modeling of Selective COX-1 Inhibitors with a Quinazoline Core. ACS Medicinal Chemistry Letters, 12(4), 610–616. https://doi.org/10.1021/acsmedchemlett.1c00004
Smith, G. F., Altman, M. D., Andresen, B., Baker, J., Brubaker, J. D., Chen, H., Chen, Y., Childers, M., Donofrio, A., Northrup, A. (2017). Identification of quinazoline based inhibitors of IRAK4 for the treatment of inflammation. Bioorganic & Medicinal Chemistry Letters, 27(12), 2721–2726. https://doi.org/10.1016/j.bmcl.2017.04.050
Cho, N.-C., Cha, J. H., Kim, H., Kwak, J., Kim, D., Seo, S.-H., Shin, Ji-S., Kim, T., Duk, K., Lee, J., No, K. T., Kim, Y. K., Lee, K.-T., Pae, A. N. (2015). Discovery of 2-aryloxy-4-amino-quinazoline derivatives as novel protease-activated receptor 2 (PAR2) antagonists. Bioorganic & Medicinal Chemistry, 23(24), 7717–7727. https://doi.org/10.1016/j.bmc.2015.11.016
Yang, S.-M., Yoshioka, М., Strovel, J. W., Urban, D. J., Hu, X., Hall, M.D., Jadhav, A., Maloney D. J. (2019). Lead optimization and efficacy evaluation of quinazoline-based BET family inhibitors for potential treatment of cancer and inflammatory diseases. Bioorg. Med Chem Lett, 29(10), 1220–1226. https://doi.org/10.1016/j.bmcl.2019.03.014
Hussein, M. A. (2013). Synthesis, anti-inflammatory, and structure antioxidant activity relationship of novel 4-quinazoline. Medicinal Chemistry Research, 22(10), 4641–4653. https://doi.org/10.1007/s00044-013-0468-9
Martynenko, Yu., Antypenko, O., Nosulenko, I., Berest, G., Kovalenko, S. (2019). Directed search of anti-inflammatory agents among (3H-quinazoline-4-ylidene)hydrazides of N-protected amino acids and their heterocyclization products. Anti-Inflammatory & Anti-Allergy Agents in Med. Chem., 19(1), 60–71. https://doi.org/10.2174/1871523018666190115092215
Krasovska, N., Stavytskyi, V., Nosulenko, I., Karpenko, O., Voskoboinik, O., Kovalenko, S. (2021). Quinazolin-containing hydrazydes of dicarboxylic acids and products of their structural modification – novel class of anti-inflammatory agents. Acta Chimica Slovenica, 68: 395–403. https://doi.org/10.17344/acsi.2020.6440.
Krasovska, N.I. (2022). Approaches to synthesis of ([1,2,4]triazolo[1,5-c]quinazolin-2-yl)benzoic acids as potential anti-inflammators. Farmatsevtychnyi zhurnal, 3, 44–54. https://doi.org/10.32352/0367-3057.3.22.05.
Voskoboynik, A. Yu., Scorina, D. Yu., Sergeieva, T. Yu., Kovalenko, S. I., Okovytyy, S. I., Omelchenko, I. V., Shishkin, O.V. (2016). Interaction of 3-(2-Aminophenyl)-6-R1-1,2,4-triazin-5-ones with Acylating Reagents: An Efficient Method for Preparation of 6-Substituted 3-R1-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones. J. Het. Chem. 5(3), 776–783. https://doi.org/10.1002/jhet.2120
Yakubovska, V. V., Seredinska, N. М., Voskoboynik, О. Yu., Stepanyuk, G. І., Kovalenko, S. І. (2016). [Purposeful search and characteristic of anti-inflammatory activity of sodium (3-R-2-оxo-2Н-[1,2,4]triazino[2,3-c]quinazolin-6-yl)alkylcarboxylates and their halogen containing analogues]. Aktualni pytannia farmatsevtychnoi i medychnoi nauky ta praktyky – Current issues in pharmacy and medicine: science and practice, 1(20), 60-66 (in Ukrainian). https://doi.org/10.14739/2409-2932.2016.1.62036.
Grib V.V., Vernigorodskiy S.V., Stepanyuk G.І. (2015). [Comparative analysis of the morphological changes of the joints when using the Sodium salt of 4- (3-methyl-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazolin-6-yl)butyric acid (compound Dsk-38) and diclofenac on adjuvant arthritis model]. Visnyk morfolohii – Reports of morphology, 21(2), 340-343 (in Ukrainian).
Grib, V. V., Doroshenko, E. N., Zaichko, N. V., Stepanyuk N. G. (2015). [Comparative characteristics of the sodium salt of of 4-(3-methyl-2-oxo-2H-[1,2,4] triazino[2,3-c]–quinazolin-6-yl)butyric acid (compound DSK-38) and diclofenac effects on hematological parameters and safety in ratswith adjuvant arthritis]. Farmakolohiia ta likarska toksykolohiia - Pharmacology and Drug Toxicology,. 6(46), 53-57 (in Ukrainian).
Balakumar, C., Lamba, P., Pran Kishore, D., Lakshmi Narayana, B., Venkat Rao, K., Rajwinder, K., Narsaiah, B. (2010). Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. European Journal of Medicinal Chemistry, 45(11), 4904–4913. https://doi.org/10.1016/j.ejmech.2010.07.063
Selvam, T.P., Kumar, P.V. (2010). Synthesis of Novel 6,7,8,9-Tetrahydro-5H-5-hydroxyphenyl-2-benzylidine-3-substituted Hydrazino Thiazolo(2,3-b)Quinazoline as Potent Antinociceptive and Anti-inflammatory Agents. Bull. Korean Chem. Soc. 31(11), 3265–3271. https://doi.org/10.5012/bkcs.2010.31.11.3265
Stavytskyi, V. V., Antypenko, O. M., Nosylenko, I. S., Berest, G. G., Voskoboinik, O. Yu., Kovalenko, S. I. (2020). Substituted 3-R-2,8-Dioxo-7,8-dihydro-2H-pyrrolo[1,2-a][1,2,4]triazino[2,3-c]quinazoline-5a(6H)carboxylic Acids and Their Salts – a Promising Class of Anti-inflammatory Agents. Anti-Inflammatory & Anti-Allergy Agents in Med. Chem., 19, 1–17. https://doi.org/ 10.2174/1871523019666200505073232.
Stavytskyi, V. V., Voskoboinik, О. Yu., Kazunin, М. S., Nosulenko, I. S., Shishkina, S., Kovalenko, S. I. (2020). Substituted pyrrolo[1,2-a][1,2,4]triazolo-([1,2,4]triazino-)[c]quinazoline-4a(5a)-propanoic acids: synthesis, spectral characteristics and anti-inflammatory activity. Voprosy khimii i khimicheskoi tekhnologii. 1, 61–70. https://doi.org/10.32434/0321-4095-2020-128-1-61-70.
Stavitskiy, V. V., Voskoboinik, O. Yu., Nosulenko, I. S., Klimova, O. O., Brazhko, O. A., Kovalenko S. I. (2019). [Substituted 3-R-7,8-dihydro-2H-pyrrolo[1,2-a][1,2,4]–triazino[2,3-c]quinazolin-5a-(6H)-alkylcarboxylic acids – promising class of low-toxic anti-inflammatory agentsv]. Farmatsevtychnyi chasopys – . Pharmaceutical review., № 3. С. 5-12 (in Ukrainian). https://doi.org/10.11603/2312-0967.2019.3.10468
Stavytskyi, V.V., Nosulenko, I.S., Kandybey, K.I., Voskoboinik, O.Yu., Kovalenko, S.I. (2020). Esters and amides of 3-R-2,8-dioxo-7,8-dihydro-2H-pyrrolo[1,2-a][1,2,4]triazino[2,3-c]quinazolin-5a(6H)-carboxylic (-propanoic) acids: synthesis and biological activity. Journal of Organic and Pharmaceutical Chemistry, 18(1), (69), 14–21. https://doi.org/10.24959/ophcj.20.192826.
Stavytskyi, V. V., Nosulenko, I. S., Portna, O. O., Shvets, V. M., Voskoboynik, O. Yu., Kovalenko, S. I. (2020). Substituted pyrrolo[1,2-a][1,2,4]triazolo-(triazino-)-[c]quinazolines - a promising class of lipoxygenase inhibitors. Current issues in pharmacy and medicine: science and practice, 13, 1(32), 4–10. https://doi.org/10.14739/2409-2932.2020.1.198109.
Poojari, S., Parameshwar Naik, P., Krishnamurthy, G., Jithendra Kumara, K.S., Sunil Kumar, N., Sathish, N. (2017) Anti-inflammatory, antibacterial and molecular docking studies of novel spiro-piperidine quinazolinone derivatives, Journal of Taibah University for Science, 11(3), 497–511, https://doi.org/10.1016/j.jtusci.2016.10.003
Kolomoets, O., Voskoboynik, О., Antypenko, O. Berest, G., Nosulenko, І., Palchikov, М., Karpenko, О., Kovalenko, S. (2017). Desing, synthesis and anti-inflammatory activity of dirivatives 10-R-3-aryl-6,7-dihydro-2H-[1,2,4]triazino[2,3-c]quinazolin-2-ones of spiro-fused cyclic frameworks. Acta Chim. Slov., 64(4), 902–910. https://doi.org/10.17344/acsi.2017.3575
Wang, N.-N., Liu, C.-Y., Wang, T., Li, Y.-L., Xu, K., Lou, H.-X. (2021). Two New Quinazoline Derivatives from the Moss Endophytic Fungus Aspergillus sp. and Their Anti-inflammatory Activity. Natural Products and Bioprospecting, 11, 105–110. https://doi.org/10.1007/s13659-020-00287-5
Duff, M. R., Gabel, S. A., Pedersen, L. C., DeRose E. F., Krahn, J. M., Howell, E. E., London, R. E. (2020). The structural basis for NSAID inhibition of human dihydrofolate reductase. J. Med. Chem. 63, 8314–8324. https://doi.org/10.1021/acs.jmedchem.0c00546
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

