DEXTRINIZATION OF STARCH WITH ALPHA-AMILASE IN THE CONDITIONS OF ETHYL ALCOHOL PRODUCTION
DOI:
https://doi.org/10.15421/jchemtech.v31i3.280974Keywords:
corn, starch, hydrolysis, enzyme preparation, alpha-аmylase, enzyme activity, sugarsAbstract
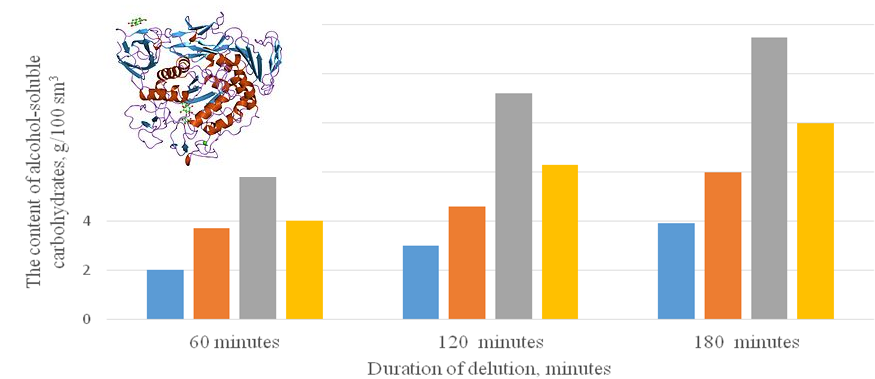
Fermentation of starch-containing raw materials in alcohol production is limited by the rate of enzymatic hydrolysis of dextrins, which depends on the productivity of alcohol per ton of raw materials. We conducted a study of the kinetics of hydrolysis of corn starch by enzymes of the amylase complex depending on their dosage in the conditions of a one-stage and two-stage dilution process. Research methods generally accepted in the alcohol industry were used in the work. Conventional starchiness was determined polarimetrically according to the Evers method. Enzymatic activity was determined by the photocolorimetric method. If the enzymatic hydrolysis of starch was carried out at a temperature of 90 °C, the loss of activity of the enzyme preparation "Amilex 4T was 73 % already after the first hour of exposure, and after three hours of exposure, the activity was only 15.4 % of the initial level. The dilution regime at temperatures of 50–70 °C for one hour and raising it to 90 °C and holding for two hours ensured the preservation of the enzyme activity, which was 62.9 % of the initial one. As a result of dilution of the wort with different dosages of the alpha-amylase enzyme, it was determined that a dose of the enzyme of 1.5 units of activity per 1 g of starch provided the necessary and sufficient level of formation of fermentable sugars. It was established that the dissolution of starch under the conditions of stepwise two-stage heating of the wort was better in comparison with the one-stage regime. The content of undissolved starch in the matured brew was 0.03 against 0.13 g/100 cm3 in the control, the indicators of unfermented sugars in the brew corresponded to the regulated ones, and the alcohol content increased by 0.2 %.
References
Hua, X., Yang, R. (2015). Enzymes in starch processing. In book Enzymes in Food and Beverage Processing. Boca Raton. CRC Press., 139–170. https://dx.doi.org/10.1201/b19408
Bednarska, K. A. (2015). Kinetic modelling of enzymatic starch hydrolysis. Wageningen: Wageningen University. https://edepot.wur.nl/343988
Dziedzic, S. Z., Kearsley, M. W. (1995). Handbook of starch hydrolysis products and their derivatives. Boston, USA: Springer
Paul, J. S., Gupta, N., Beliya, E.b., Tiwari, S., Jadhav, S. K. (2021). Aspects and Recent Trends in Microbial α-Amylase: a Review. Applied Biochemistry and Biotechnology, 193(8), 2649–2698. https://dx.doi.org/10.1007/s12010-021-03546-4
Ganti, S. M., Johnston, D. B., Rausch, K. D., Tumbleson, M. E., Singh, V. (2011) Starch hydrolysis modeling: application to fuel ethanol production. Bioprocess Biosyst Eng., 34(7), 879–890. https://dx.doi.org/10.1007/s00449-011-0539-6
Vidal, B., Rausch, K. Tumbleson, M. E., Singh, V. (2009). Kinetics of Granular Starch hydrolysis in Corn Dry-Grind Process. Starch-Starke, 61(8), 448–456. http://dx.doi.org/10.1094/CCHEM-86-2-01
Alonazi, M. K, Aida, B.-H.-a., Yacine, A., Abir Ben, B. (2021). Alpha amylase from bacillus pacificus associated with brown algae turbinaria ornata: Cultural conditions, purification, and biochemical characterization. Processes, 9(1), 1–13. https://dx.doi.org/10.3390/pr9010016.
Srichuwong, S., Sunarti, T. C., Mishima, T., Isono, N., Hisamatsu, M. (2005). Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydrate Polymers, 60(4), 529–538. https://dx.doi.org/10.1016/j.carbpol.2005.03.004
Chin, P. M., Ingledew, W. M. (1993). Effect of recycled laboratory backset on fermentation of wheat mashes. Journal of Agricultural and Food Chemistry, 41(7), 1158–1163. https://dx.doi.org/10.1021/jf00031a028
Bailey, D. F., Ollis, J. E. (1988). Biochemical Engineering Fundamentals. New York, USA: McGraw-Hill.
Bialas, W., Czerniak, A., Szymanowska-Powalowska, D. (2014). Kinetic modeling of simultaneous saccharification and fermentation of corn starch for ethanol production. Acta Biochimica Polonica, 61(1), 153–162. http://dx.doi.org/10.18388/abp.2014_1938.
Bhatia, G., Juneja, A., Johnston, D., Rausch, K., Tumbleson M. E., Singh, V. (2021). Characterization of Amylose Lipid Complexes and Their Effect on the Dry Grind Ethanol Process. Starch/Staerke, 73(7-8). doi: 10.1002/star.202100069
Vernon-Carter, E. J., Alvarez-Ramirez, J., Meraz, M., Garcia-Diaz, S. (2019). Gaining insights into alpha-amylase inhibition by glucose through mathematical modelling and analysis of the hydrolysis kinetics of gelatinized corn starch dispersions. International Journal of Biological Macromolecules, 132, 766–771. http://dx.doi.org/10.1016/j.ijbiomac.2019.03.226
Vernon-Carter, E. J., Alvarez-Ramirez, J., Bello-Perez, L. A., Reyes, I., Hernandez-Jaimes, C. (2018). Inhibition of the amylolytic hydrolysis of starch by ethanol. Food Hydrocolloids, 90, 285–290. http://dx.doi.org/10.1016/j.foodhyd.2018.12.046
Cha´vez-Camarillo, G. M., Lopez-Nuñez, P. V., Jime´nez-Nava, R. A., Aranda-Garcı´a, E., Cristiani, U. E. (2022)/ Production of extracellular α-amylase by single-stage steady-state continuous cultures of Candida wangnamkhiaoensis in an airlift bioreactor. PLoS ONE, 17(3), e0264734. https://dx.doi. org/10.1371/journal.pone.0264734
Azmi, A. S., Malek, M. I. A., Puad, N. I. M (2017). A review on acid and enzymatic hydrolyses of sago starch. International Food Research Journal, 24, 265–273. http:/www.ifrj.upm.edu.my
Al-Amri, A. A., Al-Ghamdi, M. A.a., Khan, J. A.a., Altayeb, H. N.a., Alsulami, H.A., Sajjad, M. B., Baothman, O. A. A., Nadeem? M. S. (2022)/ Escherichia coli expression and characterization of α-amylase from geobacillus thermodenitrificans dsm-465. Brazilian Journal of Biology, 82. https://dx.doi.org/10.1590/1519-6984.239449
Margetić, A., Dojnov, B., Vujčić, M., Mišić, M., Božić, N., Vujčić, Z. (2023). Modified simultaneous saccharification and fermentation for the production of bioethanol from highly concentrated raw corn starch. Fuel, 338. https://dx.doi. org/10.1016/j.fuel.2022.127363
Cripwell, R. A. a., Favaro, L., Viljoen-Bloom, M., Van Zyl, W. H. (2020). Consolidated bioprocessing of raw starch to ethanol by Saccharomyces cerevisiae: Achievements and challenges. Biotechnology Advances, 42. https://dx.doi. org/10.1016/j.biotechadv.2020.107579
Sharma, V., Rausch, K., Graeber, J., Schmidt, S., Buriak, P., Tumbleson M.E., Singh, V. (2010). Effect of resistant starch on hydrolysis and fermentation of corn starch for ethanol. Applied Biochemistry and Biotechnology, 160(3), 800–811. doi: 10.1007/s12010-009-8651-7
Polygalina, G. V. (1999). [Technochemical control of alcohol and liquor production]. Мoskva, Russian Federation: Kolos (in Russian).
Mitchell, G. A. (1990). Methods of Starch Analysis. Starch‐Stärke, 42(4), 131–134. https://dx.doi.org/10.1002/star.19900420403.
Marynchenko, V.O., Domaretskyi, V.A, Shyyan, P.L., Shvets, V.M., Tsygankov, P.S., Gholner, I.D. (2003) [Alcohol technology]. Vinnytsya, Ukraine: Podillya-2000.
SОU 15.9-37-241:2005 [Enzyme preparation for the alcohol production. Methods for determination of amylolytic activity]. Мinistеrstvo аgrarnоi pоlіtyky Ukrаiny, Кyiv. (In Ukrainian).
Albalasmeh, A. A., Berhe, A. A., Ghezzehei, T.A. (2013). A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydrate Polymers, 97(2), 253–261 https://dx.doi.org/10.1016/j.carbpol.2013.04.072https://tristan.org.ua/ru/produktsiya/fermentnye-preparaty
Langenaeken, N., De Schepper, C., De Schutter, D., Courtin, C.M. (2019). Different gelatinization characteristics of small and large barley starch granules impact their enzymatic hydrolysis and sugar production during mashing. Food Chemistry, 295, 138 – 146. doi 10.1016/j.foodchem.2019.05.045
Šokarda Slavić, M., Margetić, A., Dojnov, B., Vujčić, M., Mišić, M., Božić, N., Vujčić, Z (2023). Modified simultaneous saccharification and fermentation for the production of bioethanol from highly concentrated raw corn starch. Fuel, 338. doi 10.1016/j.fuel.2022.127363
Lugowoj, S., Balcerek, M. (2022) Polysaccharides of Starchy and Lignocellulose Materials and their Use in Ethanol Production: Enzymes and other Factors Affecting the Production Process. Applied Food Biotechnology, 9(2), 157–172. doi: 10.22037/afb.v9i2.37355
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

