CuBr2 AS A BROMINATION AGENT OF PYRAZOLE-BASED LIGAND: SYNTHESIS OF COPPER(II) COORDINATION COMPOUNDS BY OXIDATIVE DISSOLUTION OF COPPER POWDER IN ORGANIC SOLVENTS
DOI:
https://doi.org/10.15421/jchemtech.v31i3.281190Keywords:
Copper complexes, pyrazole, bromination, direct synthesis, oxidative dissolution, Hirshfeld surface analysis, crystal structure, fractional crystallizationAbstract
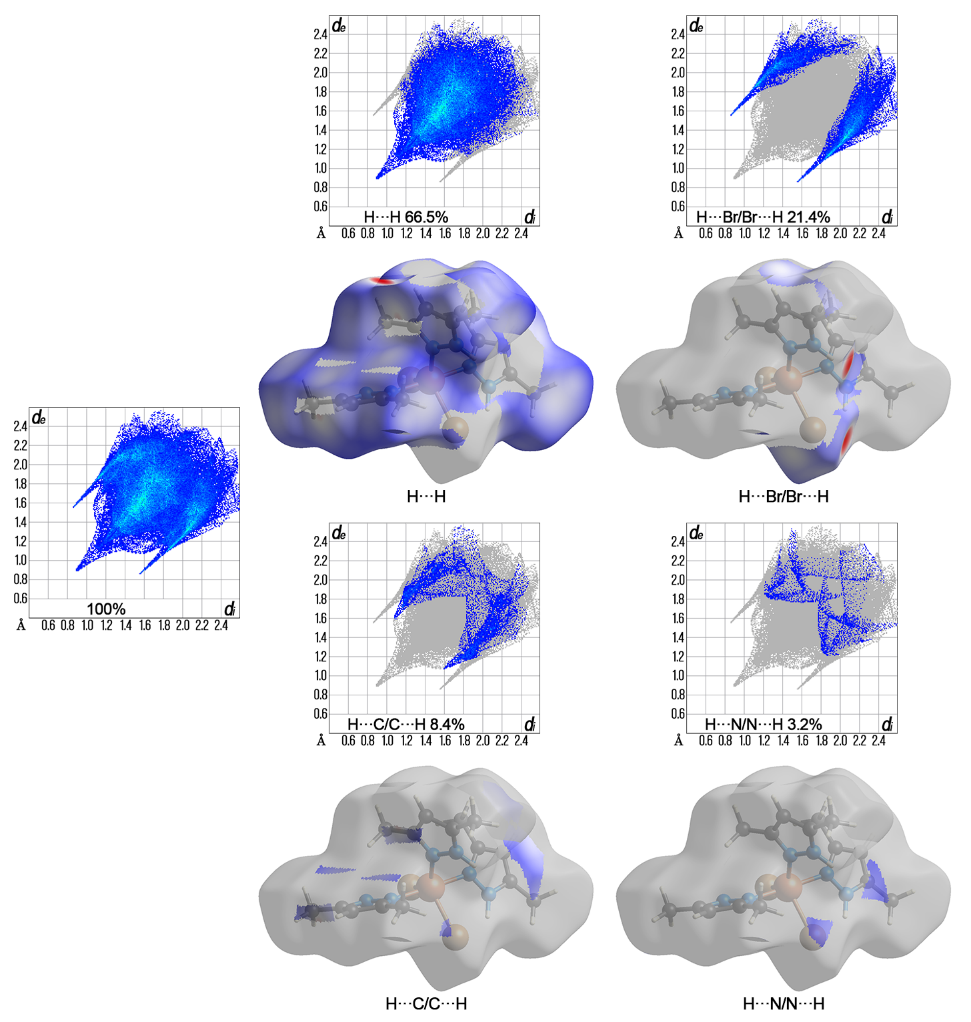
Using the direct synthesis method, by oxidative dissolution of copper powder in the presence of CuBr2 and C5H8N2 (3,5-dimethyl-1H-pyrazole), three different types of crystals were formed, isolated, and identified. Three new coordination compounds, trinuclear [Cu3(µ3-OH)(µ2-C5H7N2Br)(µ2-Br)3(C5H8N2)5Br]·CHCl3 (where C5H7N2Br – 4-bro-mo-3,5-dimethylpyrazole) (1) (orthorhombic, Pnma), binuclear [Cu2(µ2-C5H7N2Br)(µ2-Br)(C5H8N2)4Br2]·2CHCl3 (2) (monoclinic, C2/c) and mononuclear [CuBr2(C5H8N2)3] (3) (triclinic, P ), have been obtained. According to the single-crystal X-ray diffraction analysis, the complex 1 is a trinuclear six-membered cycle, where copper atoms are connected by three types of bridges: μ2-bromide ions, μ2-4-bromo-3,5-dimethylpyrazole molecule, and μ3-hydroxo group. The binuclear complex 2 is formed due to a connection between two copper atoms by a bidentate-bridging bromide ion and a bridged 4-brominated 3,5-dimethylpyrazole molecule. Coordination compound 3 is a mono-nuclear trigonal-bipyramidal copper(II) complex. During the reaction, some part of 3,5-dimethylpyrazole molecules was brominated in the 4th position of the pyrazole ring. The Hirshfeld surface analysis reveals that the inter-molecular H···H contacts have the highest contribution to the crystal packing of all compounds: 66.9 % for 1, 54.4 % for 2, and 66.5 % for 3.
References
Malinowski, J., Zych, D., Jacewicz, D., Gawdzik, B., Drzeżdżon, J. (2020). Application of Coordination Compounds with Transition Metal Ions in the Chemical Industry–A Review. International Journal of Molecular Sciences, 21(15), 5443. https://doi.org/10.3390/ijms21155443
Prier, C. K., Rankic, D. A., & MacMillan, D. W. C. (2013). Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chemical Reviews, 113(7), 5322–5363. https://doi.org/10.1021/cr300503r
Renfrew, A. K. (2014). Transition metal complexes with bioactive ligands: mechanisms for selective ligand release and applications for drug delivery. Metallomics, 6(8), 1324–1335. https://doi.org/10.1039/C4MT00069B
Haiduc, I. (2019). ReviewInverse coordination. Organic nitrogen heterocycles as coordination centers. A survey of molecular topologies and systematization. Part 1. Five-membered and smaller rings. Journal of Coordination Chemistry, 72, 2127–2159. https://doi.org/10.1080/00958972.2019.1641702
Mukherjee, R. (2000). Coordination chemistry with pyrazole-based chelating ligands: molecular structural aspects. Coordination Chemistry Reviews, 203(1), 151–218. https://doi.org/10.1016/S0010-8545(99)00144-7
Trofimenko, S. (1986). The Coordination Chemistry of Pyrazole-Derived Ligands. Progress in Inorganic Chemistry, 34, 115–210. https://doi.org/10.1002/9780470166352.ch3
Kupcewicz, B., Sobiesiak, K., Malinowska, K., Koprowska, K., Czyz, M., Keppler, B., Budzisz, E. (2012). Copper(II) complexes with derivatives of pyrazole as potential antioxidant enzyme mimics. Medicinal Chemistry Research, 22(5), 2395–2402. https://doi.org/10.1007/s00044-012-0233-5
Santra, A., Brandao, P., Jana, H., Mondal, G., Bera, P., Jana, A., Bera, P. (2018). Copper(II) and cobalt(II) complexes of 5-methyl pyrazole-3-carboxylic acid: Synthesis, X−ray crystallography, thermal analysis and in vitro antimicrobial activity. Journal of Coordination Chemistry, 71(22) 3648–3664. https://doi.org/10.1080/00958972.2018.1520984
Aljuhani, E., Aljohani, M. M., Alsoliemy, A., Shah, R., Abumelha, H. M., Saad, F. A., Hossan, A., Al-Ahmed, Z. A., Alharbi, A., El-Metwaly, N. M. (2021). Synthesis and Characterization of Cu(II)-Pyrazole Complexes for Possible Anticancer Agents; Conformational Studies as Well as Compatible in-Silico and in-Vitro Assays. Heliyon, 7(11), e08485. https://doi.org/10.1016/j.heliyon.2021.e08485
Zhou, J.-H., Liu, Z., Li, Y.-Z., Song, Y., Chen, X.-T., You, X.-Z. (2006). Synthesis, structures and magnetic properties of two copper(II) complexes with pyrazole and pivalate ligands. Journal of Coordination Chemistry, 59(2), 147–156. https://doi.org/10.1080/00958970500266206
Wiśniewski, M.Z., Głowiak, T. (2000). Copper(II) Methylpyrazole Complexes. Polish Journal of Chemistry, 74(7), 1023–1029. (1293262).
Di Nicola, C., Garau, F., Gazzano, M., Monari, M., Pandolfo, L., Pettinari, C.,Pettinari, R. (2010). Reactions of a Coordination Polymer Based on the Triangular Cluster [Cu3(μ3-OH)(μ-pz)3]2+ with Strong Acids. Crystal Structure and Supramolecular Assemblies of New Mono-, Tri-, and Hexanuclear Complexes and Coordination Polymers. Crystal Growth & Design, 10(7), 3120–3131. https://doi.org/10.1021/cg1002397
Klingele, J., Dechert, S., Meyer, F. (2009). Polynuclear transition metal complexes of metal⋯metal-bridging compartmental pyrazolate ligands. Coordination Chemistry Reviews, 253(21-22), 2698–2741. https://doi.org/10.1016/j.ccr.2009.03.026
[14] Shi, K., Mathivathanan, L., Boudalis, A. K., Turek, P., Chakraborty, I., & Raptis, R. G. (2019). Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System. Inorganic Chemistry, 58(11), 7537–7544. https://doi.org/10.1021/acs.inorgchem.9b00748
Bala, S., Akhtar, S., Liu, J.-L., Huang, G.-Z., Wu, S.-G., De, A., Das, K. S., Saha, S., Tong, M.-L., Mondal, R. (2021). Fascinating Interlocked Triacontanuclear Giant Nanocages. Chemical Communications, 57(85), 11177–11180. https://doi.org/10.1039/D1CC02990H
Sarma, P., Sharma, P., Frontera, A., Barcelo-Oliver, M., Verma, A. K., Barthakur, T., & Bhattacharyya, M. K. (2021). Unconventional π-hole and Semi-coordination regium bonding interactions directed supramolecular assemblies in pyridinedicarboxylato bridged polymeric Cu(II) Compounds: Antiproliferative evaluation and theoretical studies. Inorganica Chimica Acta, 525, 120461. https://doi.org/10.1016/j.ica.2021.120461
Castro, I., Calatayud, M.L., Orts-Arroyo, M., Moliner, N., Marino, N., Lloret, F., Ruiz-García, R., Munno, G.D., Julve, M. (2021). Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives. Molecules, 26, 2792. https://doi.org/10.3390/molecules26092792
Kokozay, V. N.; Vassilyeva, O. Y.; Makhankova, V. G. (2018). Direct Synthesis of Heterometallic Complexes. In Direct Synthesis of Metal Complexes; Kharisov, B., Ed.; Elsevier, 183–237. https://doi.org/10.1016/B978-0-12-811061-4.00005-0
Li, X., Binnemans, K. (2021). Oxidative Dissolution of Metals in Organic Solvents. Chemical Reviews, 121(8), 4506–4530. https://doi.org/10.1021/acs.chemrev.0c00917
Swain, S. P., Kumar, K. N., Mhate, M., Panchami, H., Ravichandiran, V. (2022) Copper (II) Bromide Catalysed One Pot Bromination and Amination for the Green, Cost-Effective Synthesis of Clopidogrel. Molecular Catalysis, 522, 112210. https://doi.org/10.1016/j.mcat.2022.112210
Zai, Y., Feng, Y., Zeng, X., Tang, X., Sun, Y., Lin, L. (2019). Synthesis of 5-aminolevulinic acid with nontoxic regents and renewable methyl levulinate. RSC Advances, 9(18), 10091–10093. https://doi.org/10.1039/C9RA01517E
Moldoveanu, C., Amariucai-Mantu, D., Mangalagiu, V., Antoci, V., Maftei, D., Mangalagiu, I. I., Zbancioc, G. (2019). Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules, 24(20), 3760. https://doi.org/10.3390/molecules24203760
Porré, M., Pisanò, G., Nahra, F., Cazin, C.S.J. (2022). Synthetic Access to Aromatic α-Haloketones. Molecules, 27,3583. https://doi.org/10.3390/molecules27113583
Akhtar, R., Zahoor, A. F., Rasool, N., Ahmad, M., Ali, K. G. (2021). Recent trends in the chemistry of Sandmeyer reaction: a review. Molecular Diversity, 26(3), 1837–1873. https://doi.org/10.1007/s11030-021-10295-3
Rigaku O. D. (2019). CrysAlisPRO Software system (ver. 1.171.40.53); Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England.
Sheldrick, G. M. (2015). SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallographica Section A, 71(1), 3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick G. M. (2015). Crystal structure refinement with SHELXL. Acta Crystallographica Section C, 71, 3–8. https://doi.org/10.1107/S2053229614024218
Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H. (2009). OLEX2: a complete structure solution, refinement and analysis program. Journal of Applied Crystallography, 42, 339–341. https://doi.org/10.1107/S0021889808042726
Clark, R. C., Reid, J. S. (1995). The analytical calculation of absorption in multifaceted crystals. Acta Crystallographica Section A, 51(6), 887–897. https://doi.org/10.1107/S0108767395007367
Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. Spackman, M. A. (2021). CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Journal of Applied Crystallography, 54(3), 1006–1011. https://doi.org/10.1107/S1600576721002910
Sundaraganesan, N., Kavitha, E., Sebastian, S., Cornard, J. P., Martel, M. (2009). Experimental FTIR, FT-IR (gas phase), FT-Raman and NMR spectra, hyperpolarizability studies and DFT calculations of 3,5-dimethylpyrazole. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 74(3), 788–797. https://doi.org/10.1016/j.saa.2009.08.019
Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., Verschoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper (II) perchlorate. Journal of the Chemical Society, Dalton Transactions, (7), 1349–1356. https://doi.org/10.1039/DT9840001349
Wei, W., Xu, Y. (2012). (μ-4-Bromo-3,5-dimethylpyrazolato-κ2N1:N2)-μ-chlorido-bis[bis(4-bromo-3,5-dimethylpyrazole-κN2)chloridocopper(II)] acetonitrile monosolvate. Acta Crystallographica Section E Structure Reports Online, 68(5), m557. https://doi.org/10.1107/S160053681201402X.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

