MODELING OF ZIRCONIUM(IV) METHANESULFONATE AND SULFATE COMPLEXES IN AQUEOUS SOLUTION
DOI:
https://doi.org/10.15421/jchemtech.v31i3.282043Keywords:
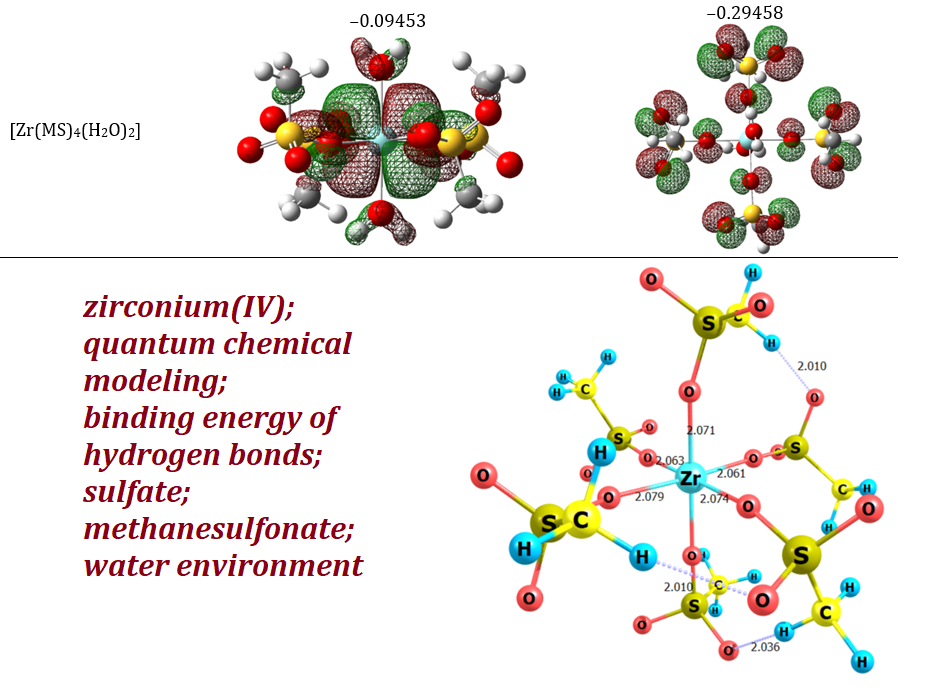
zirconium(IV), quantum chemical modeling, binding energy of hydrogen bonds, sulfate, methanesulfonate, water environmentAbstract
The article theoretically investigated the features of the geometric and electronic structure of zirconium(IV) methanesulfonate and sulfate complexes in aqueous solution. The interatomic distances of the central ion with the surrounding ligands are considered in detail. It is shown what geometric and electronic structure zirconium(IV) sulfate and methanesulfonate complexes can have under the influence of an aqueous environment and the mutual influence of surrounding ligands. As a result, interatomic distances, torsion and valence angles were described in detail. The sum of electronic and zero-point energies and to the thermal free energies are calculated and shown. The frontier molecular orbitals (HOMO and LUMO) have been calculated and as a result it was demonstrated that the stability of the studied compounds decreases in the order [Zr(MS)6]2– > [ZrO(MS)2] > [ZrO(MS)2(H2O)4] > [Zr(MS)4(H2O)2] > [ZrO(SO4)2(H2O)]2– >[ZrO(SO4)2]2–. Furthermore, it have been established that most complexes of zirconium(IV) with methanesulfonate and sulfate anions can form intramolecular hydrogen bonds, in complexes such as [ZrO(SO4)2(H2O)]2–, [ZrO(MS)2(H2O)4], [Zr(MS)6]2– and [ZrO(MS)2]. It should be noted that, in addition to the known complexes [ZrO(MS)2(H2O)4] and [ZrO(SO4)2]2–, the possibility of the existence of complexes [Zr(MS)6]2– and [ZrO(SO4)2(H2O)]2– is shown in aqueous solution.
References
Gevorkyan, E., Nerubatskyi, V., Chyshkala, V., Morozova, O. (2021). Revealing specific features of structure formation in composites based on nanopowders of synthesized zirconium dioxide. Eastern-European Journal of Enterprise Technologies, 5(12), 113. https://doi.org/10.15587/1729-4061.2021.242503
Vasylkiv, O. O., Sakka, Y., & Skorokhod, V. V. (2005). Features of preparing nano-size powders of tetragonal zirconium dioxide stabilized with yttrium. Powder Metallurgy and Metal Ceramics, 44, 228–239. https://doi.org/10.1007/s11106-005-0086-2
Togatorop, E., Suzuki-Muresan, T., Harto, A. W. (2022). A review on the solubility of crystalline zirconium dioxide and thorium dioxide. In AIP Conference Proceedings, 2501(1), 030002. https://doi.org/10.1063/5.0093941
Bocanegra-Bernal, M. H., De La Torre, S. D. (2002). Phase transitions in zirconium dioxide and related materials for high performance engineering ceramics. Journal of materials science, 37, 4947–4971. https://doi.org/10.1023/A:1021099308957
Patil, N. A., Kandasubramanian, B. (2020). Biological and mechanical enhancement of zirconium dioxide for medical applications. Ceramics International, 46(4), 4041–4057. https://doi.org/10.1016/j.ceramint.2019.10.220
Lee, M., Han, S. I., Kim, C., Velumani, S., Han, A., Kassiba, A. H., Castaneda, H. (2022). ZrO2/ZnO/TiO2 nanocomposite coatings on stainless steel for improved corrosion resistance, biocompatibility, and antimicrobial activity. ACS Applied Materials & Interfaces, 14(11), 13801–13811. https://doi.org/10.1021/acsami.1c19498
Jiang, L., Liao, Y., Wan, Q., Li, W. (2011). Effects of sintering temperature and particle size on the translucency of zirconium dioxide dental ceramic. Journal of Materials Science: Materials in Medicine, 22, 2429–2435. https://doi.org/10.1007/s10856-011-4438-9
Pekkan, G., Pekkan, K., Bayindir, B. Ç., Özcan, M., Karasu, B. (2020). Factors affecting the translucency of monolithic zirconia ceramics: A review from materials science perspective. Dental materials journal, 39(1), 1–8. https://doi.org/10.4012/dmj.2019-098
Kozakiewicz, M., Gmyrek, T., Zajdel, R., Konieczny, B. (2021). Custom-made zirconium dioxide implants for craniofacial bone reconstruction. Materials, 14(4), 840. https://doi.org/10.3390/ma14040840
Vereschak, V. G., Baskevich, A. S., Brodnikoskyi, E. M. (2018). Production of stabilized zirconia from heterometalic methanesulfonate complexes of zirconium(IV). Voprosy khimii i khimicheskoi tekhnologi, 6, 5–11. https://doi.org/10.32434/0321-4095-2018-121-6-5-11
Brykala, M., Walczak, R., Wawszczak, D., Kilim, S., Rogowski, M., Strugalska-Gola, E., Szuta, M. (2021). Preparation by the double extraction process with preliminary neutron irradiation of yttria or calcia stabilised cubic zirconium dioxide microspheres. Nuclear Engineering and Technology, 53(1), 188–198. https://doi.org/10.1016/j.net.2020.06.032
Komissarenko, D. A., Sokolov, P. S., Evstigneeva, A. D., Slyusar, I. V., Nartov, A. S., Volkov, P. A., Dosovitsky, A. E. (2021). DLP 3D printing of scandia-stabilized zirconia ceramics. Journal of the European Ceramic Society, 41(1), 684–690. https://doi.org/10.1016/j.jeurceramsoc.2020.09.010
Frisch, M. J. E. A., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Fox, A. D. (2009). Gaussian 09, Revision D.01. Gaussian. Inc., Wallingford.
König, F. B., Schönbohm, J., Bayles, D. (2001). AIM2000-a program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Becke, A. D. (1993). Density-Functional Thermochemistry. III. The Role of Exact Exchange. Indian Journal of Pure & Applied Physics, 98(7), 5648–5656. https://doi.org/10.1063/1.464913
Lee, C., Yang, W., Parr, R. G. (1988). Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical review B, 37(2), 785. https://doi.org/10.1103/PhysRevB.37.785
Pritchard, B. P., Altarawy, D., Didier, B., Gibson, T. D., Windus, T. L. (2019). New basis set exchange: An open, up-to-date resource for the molecular sciences community. Journal of chemical information and modeling, 59(11), 4814–4820. https://doi.org/10.1021/acs.jcim.9b00725.
Rao, N., Holerca, M. N., Klein, M. L., Pophristic, V. (2007). Computational study of the Zr4+ tetranuclear polymer,[Zr4(OH)8(H2O)16]8+. The Journal of Physical Chemistry A, 111(45), 11395–11399. https://doi.org/10.1021/jp0734880
He, C., Chen, Y., & Sheng, Y. (2019). First-principles study of molecular hydrogen adsorption on MgnZr (n = 1 ~ 11) clusters. The European Physical Journal D, 73, 1–8. https://doi.org/10.1140/epjd/e2019-90521-6
Stern, R. D., Kingsbury, R. S., Persson, K. A. (2021). Aqueous Stability of Zirconium Clusters, Including the Zr(IV) Hexanuclear Hydrolysis Complex [Zr6O4(OH)4(H2O)24]12+, from Density Functional Theory. Inorganic Chemistry, 60(20), 15456–15466. https://doi.org/10.1021/acs.inorgchem.1c02078
Barone, V., Cossi, M., Tomasi, J. (1998). Geometry optimization of molecular structures in solution by the polarizable continuum model. Journal of Computational Chemistry, 19(4), 404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Tomasi, J., Mennucci, B., Cammi, R. (2005). Quantum mechanical continuum solvation models. Chemicalreviews, 105(8), 2999–3094. https://doi.org/10.1021/cr9904009
Bader, R. F. (1985). Atomsinmolecules. Accounts of Chemical Research, 18(1), 9–15. https://doi.org/10.1021/ar00109a003
Espinosa, E., Molins, E., Lecomte, C. (1998). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical Physics Letters, 285(3/4), 170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Weinhold, F., Landis, C. R. (2001). Natural bond orbitals and extensions of localized bonding concepts. Chemistry Education Research and Practice, 2(2), 91–104. https://doi.org/10.1039/B1RP90011K
Rudnev, V. S., Yarovaya, T. P., Nedozorov, P. M., Ustinov, A. Y., Tyrina, L. M., Malyshev, I. V., Gnedenkov, S. V. (2011). Obtaining ZrO2+CeOx+TiO2/Ti compositions by plasma-electrolytic oxidation of titanium and investigating their properties. Protection of Metals and Physical Chemistry of Surfaces, 47, 621–628. https://doi.org/10.1134/S2070205111050145
Vereshchak, V. (2018). Obtaining, studying the properties, and application of zirconium(IV) oxymethanesulfonate. Eastern-European Journal of Enterprise Technologies, 6(6), 96, 14–19. https://doi.org/10.15587/1729-4061.2018.150771.
Khrupchyk, E. S., Pasenko, O. O., Vereshchak, V. H., Osokin, Y. S. (2023). Peculiarities of the electronic structure of the complex [ZrO(CH3SO3)2(H2O)4] in aqueous solution. 249–250. https://dspace.nuph.edu.ua/bitstream/123456789/30321/1/%D0%97%D0%B1%D1%96%D1%80%D0%BD%D0%B8%D0%BA%2018.05.2023%2B%D0%BE%D0%B1%D0%BA%D0%BB%D0%B0%D0%B4%D0%B8%D0%BD%D0%BA%D0%B0%20%281%29.pdf#page=250
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

