QUANTUM CHEMICAL STUDY OF THE STERIC EFFECT OF SUBSTITUENT ON THE REACTIVITY OF TERTIARY AMINES IN THE REACTION WITH 2-(CHLOROMETHYL)OXIRANE
DOI:
https://doi.org/10.15421/jchemtech.v31i3.283788Keywords:
epichlorohydrin, tertiary amines, ring opening, transition state, steric effect, quantum chemical modelingAbstract
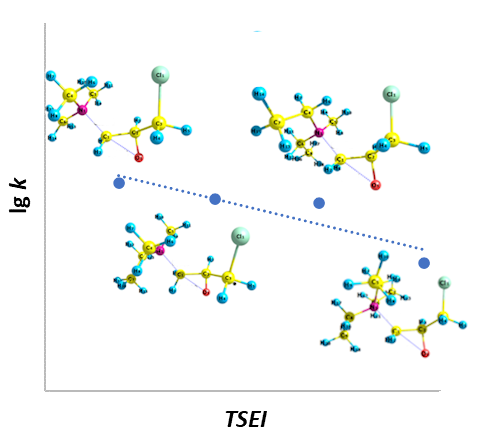
The ring-opening reaction of 2-(chloromethyl)oxirane (epichlorohydrin) with tertiary amines was investigated using the ab initio method in the gas phase. A series of amines, MenEt3–nN, with varying steric factors was selected. Geometric parameters and the order of bond breaking and formation were established for localized transition states along the reaction pathway. Energy profiles for the quaternization reaction between amines and epoxide were obtained, and the activation parameters of the process were calculated. The influence of the hydrocarbon radical length in the amine molecule on the energy barrier and the reaction rate was determined. It was demonstrated that the rate of quaternization is determined by charge control. The dissociative nature of transition states was demonstrated via More O'Ferrall–Jenks plots. Correlation equations were derived to relate steric effects and nucleophilicity of amines to their reactivity with 2-(chloromethyl)oxirane.
References
Kasian, L. I., Kasian, A. O., Okovityi, S. I., Tarabara, I. N. (2003). [Alicyclic epoxy compounds. Reactivity]. Dnipropetrovsk, Ukraine: Izdatelstvo Dnepropetrovskogo universiteta (in Russian).
Fallah-Mehrjardi, M., Kiasat, A. R., Niknam, K. (2018). Nucleophilic ring-opening of epoxides: trends in β-substituted alcohols synthesis. J. Iran. Chem. Soc., 15(9), 2033–2081. doi: 10.1007/s13738-018-1400-5.
Moschona, F., Savvopoulou, I., Tsitopoulou, M., Tataraki, D., Rassias, G. (2020). Epoxide Syntheses and Ring-Opening Reactions in Drug Development. Catalysts, 10(10), 1117–1181. doi: 10.3390/catal10101117.
Noble, J. M., Chang, L., Chen, D., Wang, B., Dominey, R. N., Cook, D. W., Burns, J. M., Stringham, R. W., Cardoso, F. S. P., Snead, D. R. (2022). A Practical and Economical Route to (S)-Glycidyl Pivalate. SynOpen, 6(4), 258–262. doi: 10.1055/s-0042-1751375.
Karami, Z., Kabiri, K., Zohuriaan-Mehr, M. J. (2019). Non-isocyanate polyurethane thermoset based on a bio-resourced star-shaped epoxy macromonomer in comparison with a cyclocarbonate fossil-based epoxy resin: A preliminary study on thermo-mechanical and antibacterial properties. J. CO2 Util., 34(4), 558–567. doi: 10.1016/j.jcou.2019.08.009.
Singh, G. S., Mollet, K., D’Hooghe, M., De Kimpe, N. (2013) Epihalohydrins in organic synthesis. Chem. Rev., 113(3), 1441–1498. doi: 10.1021/cr3003455.
Herzberger, J., Niederer, K., Pohlit, H., Seiwert, J., Worm, M., Wurm, F. R., Frey, H. (2016). Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev., 116(4), 2170–2243. doi: 10.1021/acs.chemrev.5b00441.
Yan, Z., Wang, Y., Du, C., Deng, J., Luo, G. (2022). Highly efficient two‐stage ring‐opening of epichlorohydrin with carboxylic acid in a microreaction system. AIChE J., 68(11), e17791. doi: 10.1002/aic.17791.
Bespalko, Y., Sinel’nikova, M., Shved, E., Bakhalova, E. (2021). Experimental and computational studies of the mechanism of base-catalyzed ring opening of 2-(chloromethyl)oxirane by benzoic acid. Int. J. Chem. Kinet., 53(3), 356–368. doi: 10.1002/kin.21448.
Bakhtin, S., Shved, E., Bespal’ko, Y. (2017). Nucleophile – electrophile interactions in the reaction of oxiranes with carboxylic acids in the presence of tertiary amines. J. Phys. Org. Chem., 30(12), e3717. doi: 10.1002/poc.3717.
Yutilova, K., Shved, E., Chervonchenko, I. (2022). Ukraine Patent No. 152096. Vinnytsia, Ukraine. Vasyl’ Stus Donetsk National University.
Shields, E. S., Merrill, G. N. (2007). A computational study into the reactivity of epichlorohydrin and epibromohydrin under acidic conditions in the gas phase and aqueous solution. J. Phys. Org. Chem., 20, 1058–1071. doi: 10.1002/poc.1255.
Li., J., Yu, X. Z., Zhang, K. (2011). Analysis of Ring-Opening Reaction between Bisphenol A and Epichlorohydrin by the Method of Quantum Chemical Calculating. Adv. Mater. Res., 221, 180–183. doi: 10.4028/www.scientific.net/AMR.221.180.
Pham, M. P., Pham, B. Q., Huynh, L. K., Pham, H. Q., Marks, M. J., Truong, T. N. (2014). Density functional theory study on mechanisms of epoxy‐phenol curing reaction. J. Comput. Chem., 35(22), 1630–1640. doi: 10.1002/jcc.23658.
Ly, U. Q., Pham, M. P., Marks, M. J., Truong, T. N. (2017). Density functional theory study of mechanism of epoxy-carboxylic acid curing reaction. J. Comput. Chem., 38(14), 1093–1102. doi: 10.1002/jcc.24779.
Bespalko, Y. N., Shved, E. N. (2019). Experimental and theoretical study on the kinetics and mechanism of the amine-catalyzed reaction of oxiranes with carboxylic acids. React. Kinet. Mech. Catal., 126(2), 903–919. doi: 10.1007/s11144-018-01524-2.
Persson, J., Berg, U., Matsson, O. (1995). Steric Effects in SN2 Reactions. Primary Carbon Kinetic Isotope Effects in Menshutkin Reactions. J. Org. Chem., 60(16), 5037–5040. doi: 10.1021/jo00121a024.
Yutilova, K., Bakhtin, S., Shved, E., Bespalko, Y., (2015). Tertiary amines nucleophilicity in quaternization reaction with benzyl chloride. Visnik Dnipropetrovs’kogo universitetu. Seria himia, 23(2), 15–21. doi: 10.15421/081513
K., Bakhtin, S., Bespalko, Y., Shved, E. (2016). Catalytic activity of tertiary amines with antisymbatic change of basic and nucleophilic properties in the chloroxypropylation reaction of acetic acid. React. Kinet. Mech. Catal., 119(1), 139–148. doi: 10.1007/s11144-016-1051-4.
Banks, H. D., White, W. E. (2001). A computational study of the reactions of thiiranes with ammonia and amines. J. Org. Chem., 66(18), 5981–5986. doi: 10.1021/jo001719s.
Anslyn, E. V., Dougherty, D. A. (2006). Modern physical organic chemistry. Sausalito, USA: University Science.
Johnson, C. D. (1980). The Hammett Equation. Cambridge, UK: Cambridge University Press.
Cao, C., Liu, L. (2004). Topological Steric Effect Index and Its Application. J. Chem. Inf. Comput. Sci., 44(2), 678–687. doi: 10.1021/ci034266b.
MarvinSketch v. 23.2. ChemAxon. https://chemaxon.com/products/marvin.
Jennings, E. V., Nikitin, K., Ortin, Y., Gilheany, D. G. (2014). Degenerate nucleophilic substitution in phosphonium salts. J. Am. Chem. Soc., 136(46), 16217–16226. doi: 10.1021/ja507433g.
Kalu, G. I., Ubochi, C. I., Onyido, I. (2022). Mapping transition state structures for thiophosphinoyl group transfer between oxyanionic nucleophiles in water and aqueous ethanol solvents. New J. Chem., 46(27), 12981–12993. doi: 10.1039/D2NJ02008D.
Chen, S. (2008). Quantum Chemical Modeling of Binuclear Zinc Enzymes (Doctoral dissertation). https://www.diva-portal.org/smash/get/diva2:127039/FULLTEXT01.pdf
Yoh, S. D., Cheong, D. Y., Lee, O. S. (2003). Quantitative approach to the Menschutkin reaction of benzylic systems. J. Phys. Org. Chem., 16(1), 63–68. doi: 10.1002/poc.574.
Williams, A. (2003). Free Energy Relationships in Organic and Bio-organic Chemistry. Cambridge, UK: The Royal Society of Chemistry.
Barca, G. M. J., Bertoni, C., Carrington, L., Datta, D., De Silva, N., Deustua, J. E., Fedorov, D. G., Gour, J. R., Gunina, A. O., Guidez, E., Harville, T., Irle, S., Ivanic, J., Kowalski, K., Leang, S. S., Li, H., Li, W., Lutz, J. J., Magoulas, I., Mato, J., Mironov, V., Nakata, H., Pham, B. Q., Piecuch, P., Poole, D., Pruitt, S. R., Rendell, A. P., Roskop, L. B., Ruedenberg, K., Sattasathuchana, T., Schmidt, M. W., Shen, J., Slipchenko, L., Sosonkina, M., Sundriyal, V., Tiwari, A., Galvez V., Jorge L., Westheimer, B., Włoch, M., Xu, P., Zahariev, F., Gordon, M. S. (2020). Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys., 152(15), 154102. doi: 10.1063/5.0005188.
Depizzol, D. B., Paiva, M. H. M., Dos Santos, T. O., Gaudio, A. C. (2005). MoCalc: A new graphical user interface for molecular calculations. J. Comput. Chem., 26(2), 142–144. doi: 10.1002/jcc.20151.
Scott, A. P., Radom, L. (1996). Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Møller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem., 100(41), 16502–16513. doi: 10.1021/jp960976r.
Bouteiller, Y., Gillet, J. C., Gregoire, G., Schermann, J. P. (2008). Transferable specific scaling factors for interpretation of infrared spectra of biomolecules from density functional theory. J. Phys. Chem. A, 112(46), 11656–11660. doi: 10.1021/jp805854q.
Govender, A., Curulla Ferré, D., Niemantsverdriet, J. W. H. (2012). A Density Functional Theory Study on the Effect of Zero-Point Energy Corrections on the Methanation Profile on Fe(100). ChemPhysChem, 13(6), 1591–1596. doi: 10.1002/cphc.201100733.
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

