FLUORESCENCE-BASED QUANTIFICATION OF PEPTIDE ADSORPTION ON TITANIUM DIOXIDE
DOI:
https://doi.org/10.15421/jchemtech.v31i3.284204Keywords:
titanium dioxide, adsorption, fluorescence, peptideAbstract
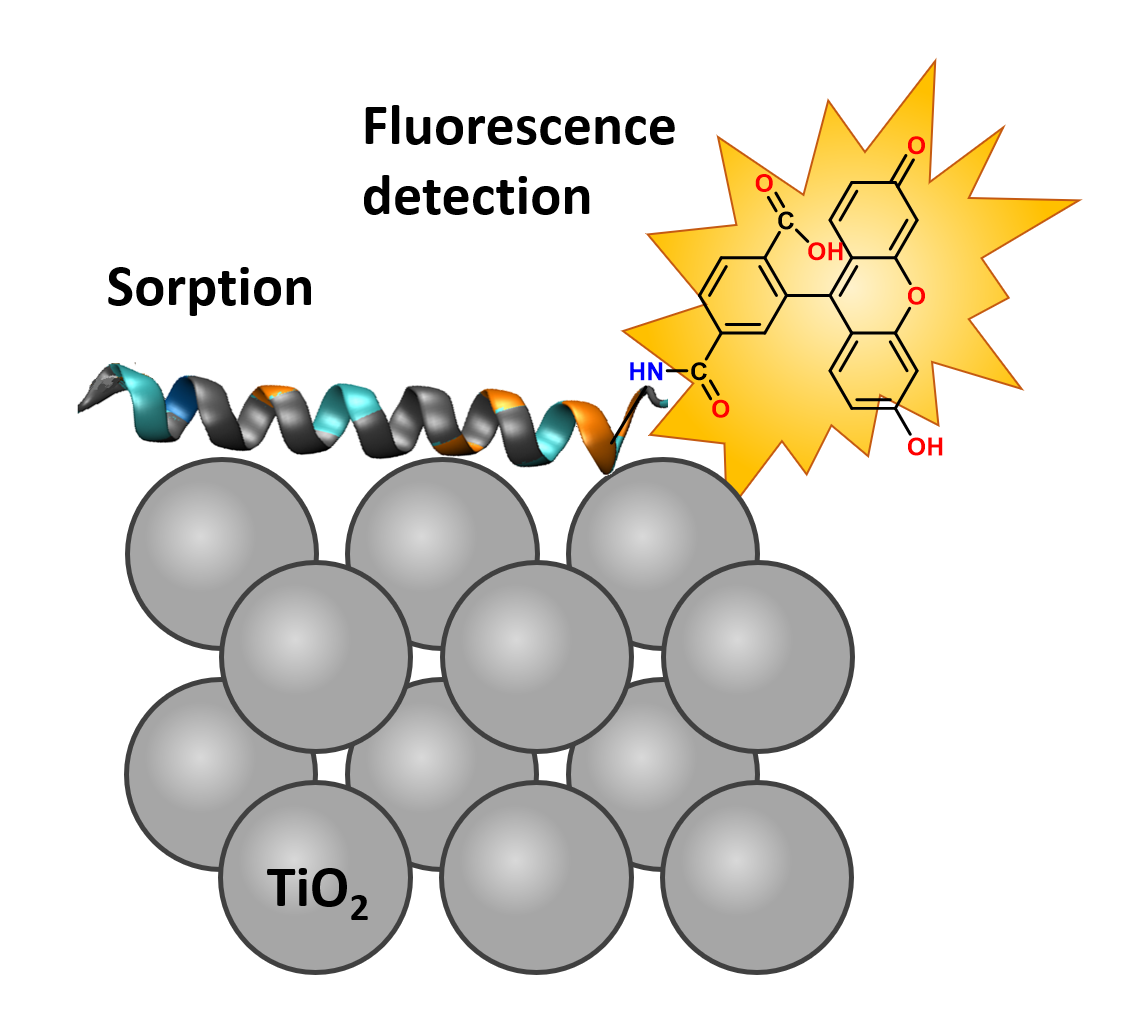
Titanium dioxide is widely used as a white colorant in medicines, cosmetics, and in the food industry due to the absence of taste and smell, chemical stability in combination with excellent ultraviolet protection properties. However, its safety as a food additive was recently questioned by the European Food Safety Authority (EFSA). There is a need to deeper study the interaction of TiO2 with the human body and biological tissues. Proteins are the main type of biological molecules that can interact with TiO2 after oral administration of TiO2-containing drugs. The classical approach to measuring adsorption of organic molecules on TiO2 is based on spectrophotometry. However, the sensitivity of this method is not sufficient for measurements of the adsorption of peptides and proteins. This problem can be bypassed using peptides covalently labeled with fluorescent organic dyes possessing relatively high molar absorption coefficients and long absorption wavelengths. In this work, we decided to test the applicability of fluorescence-based quantification of peptide adsorption on titanium dioxide. Namely, we used 11 amino acids peptide labeled with fluorescein and quantified its adsorption on TiO2 at different ionic strengths of the solution. There are several advantages of using the fluorescence method to study the binding of peptides to a sorbent surface. The use of low peptide concentrations and small sample volumes allows for efficient use of resources. Reliable readout, fast measurement time, and cost-effectiveness make this method attractive for the future peptide binding studies and could potentially find application in other areas of peptide research.
References
Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171). https://eur-lex.europa.eu/eli/reg/2022/63/oj
EMA. (2021). Final feedback from European Medicine Agency (EMA) to the EU Commission request to evaluate the impact of the removal of titanium dioxide from the list of authorised food additives on medicinal products. European Medicines Authority. EMA/504010/2021. https://www.ema.europa.eu/en/documents/report/final-feedback-european-medicine-agency-ema-eu-commission-request-evaluate-impact-removal-titanium_en.pdf
Enciso, M., Schütte, C., Delle Site, L. (2015). Influence of pH and sequence in peptide aggregation via molecular simulation. The Journal of chemical physics, 143(24), 243130. https://doi.org/10.1063/1.4935707
Mironyuk, I., Vasylyeva, H., Prokipchuk, I., Mykytyn, I. (2023). Adsorption of Sr(II) cations onto titanium dioxide, doped with Boron atoms. Physics and Chemistry of Solid State, 24(1), 114–125. https://doi.org/10.15330/pcss.24.1.114-125
Nelson, D. L., Cox, M. M. (2017). Lehninger principles of biochemistry (7th ed.). W.H. Freeman.
Barberi, J., Spriano, S. (2021). Titanium and protein adsorption: An overview of mechanisms and effects of surface features. Materials, 14(7), 1590. https://doi.org/10.3390/ma14071590
Mironyuk, I., Danyliuk, N., Tatarchuk, T., Mykytyn, I., Kotsyubynsky, V. (2021). Photocatalytic degradation of Congo red dye using Fe-doped TiO2 nanocatalysts. Physics and Chemistry of Solid State, 22(4), 697–710. https://doi.org/10.15330/pcss.22.4.697-710
Şişmanoğlu, T., Pozan, G. S. (2016). Adsorption of congo red from aqueous solution using various TiO2 nanoparticles. Desalination and Water Treatment, 57(28), 13318–13333. https://doi.org/10.1080/19443994.2015.1056834
Mironyuk, I., Kotsyubynsky, V., Mykytyn, I., Kotsyubynska, Y., Boychuk, V., Fedoriv, V. (2021). Iron-doped Titania: Synthesis, structural, magnetic and photocatalytic properties. Journal of Nano- and Electronic Physics, 13(6), 06023-1-06023–06027. https://doi.org/10.21272/jnep.13(6).06023
Mironyuk, I. F., Soltys, L. M., Tatarchuk, T. R., Savka, K. O. (2020). Methods of titanium dioxide synthesis (review). Physics and Chemistry of Solid State, 21(3), 462–477. https://doi.org/10.15330/pcss.21.3.462-477
Mironyuk, I., Danyliuk, N., Turovska, L., Mykytyn, I. (2022). Structural, morphological and photocatalytic properties of TiO2 obtained by thermolytic decomposition of the [Ti(OH2)6]3+•3Cl¯ aquacomplex. Physics and Chemistry of Solid State, 23(4), 741–755. https://doi.org/10.15330/pcss.23.4.741-755
Zamotaiev, O. M., Postupalenko, V. Y., Shvadchak, V. V., Pivovarenko, V. G., Klymchenko, A. S., Mély, Y. (2014). Monitoring penetratin interactions with lipid membranes and cell internalization using a new hydration-sensitive fluorescent probe. Organic & Biomolecular Chemistry, 12(36), 7036–7044. https://doi.org/10.1039/c4ob01242a
Ladokhin, A. S. (2000). Fluorescence Spectroscopy in Peptide and Protein Analysis. In Encyclopedia of Analytical Chemistry. John Wiley & Sons, Ltd. https://doi.org/10.1002/9780470027318.a161
Abin-Bazaine, A., Campos Trujillo, A., & Olmos-Marquez, M. (2022). Adsorption isotherms: Enlightenment of the phenomenon of adsorption. In Wastewater Treatment. IntechOpen. https://doi.org/10.5772/intechopen.104260
Karp, G., Iwasa, J., Marshall, W. (2020). Karp’s Cell and Molecular Biology. 9th Ed. New Jersey: John Wiley & Sons, Inc.
Wang, J., Guo, X. (2020). Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere, 258(127279), 127279. https://doi.org/10.1016/j.chemosphere.2020.127279
Mironyuk, I., Myslin, M., Lapchuk, I., Tatarchuk, T., & Olkhovyy, O. (2021). Adsorption of azo dye Congo red on the Sn-doped TiO2 surface. Physics and Chemistry of Solid State, 22(3), 561–567. https://doi.org/10.15330/pcss.22.3.561-567
Musah, M., Azeh, Y., Mathew, J., Umar, M., Abdulhamid, Z., Muhammad, A. (2022). Adsorption kinetics and isotherm models: A review. Caliphate Journal of Science and Technology, 4(1), 20–26. https://doi.org/10.4314/cajost.v4i1.3
Piergiovanni, P. (2020). Introductory Adsorption Laboratory Experiment. 2012 ASEE Annual Conference & Exposition Proceedings. https://doi.org/10.18260/1-2--21610
Foo, K. Y., Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal (Lausanne, Switzerland: 1996), 156(1), 2–10. https://doi.org/10.1016/j.cej.2009.09.013
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

