COMPLEX RESEARCH OF NEW ADSORPTION MATERIALS
DOI:
https://doi.org/10.15421/jchemtech.v32i1.284703Keywords:
adsorption; coconut coal; coagulants; flocculants; iron; manganese.Abstract
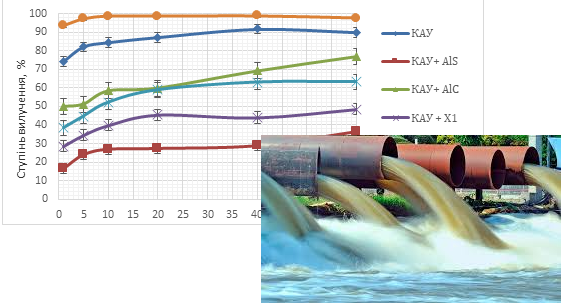
In this work, a comprehensive comparative study of adsorbents obtained by combining coconut coal with coagulants based on aluminium salts (Al2(SO4)3 and AlCl3) and flocculants (sodium alginate and chitosan) was obtained and carried out for the removal of iron and manganese ions from model solutions. Adsorbents based on coconut coal and flocculants were obtained by the impregnation method followed by drying at a temperature of 50 °C for 6 hours (КАУ+Х1, КАУ+Х2 and КАУ+АН). Adsorbents based on coconut coal and coagulants were produced by adding aluminum salts to an aqueous solution of urea at a temperature of 95 °C with intensive stirring for 30 minutes using coconut coal (КАУ+ AlC КАУ+AlS). The adsorbents were tested for their ability to remove iron and manganese ions from water systems. The comparative characteristics were based on the results of the adsorption kinetics study. It was found that the most effective adsorbent for removing iron and manganese ions is based on coconut coal and sodium alginate flocculant (КАУ+АН), in comparison with coconut coal alone. The recommended contact duration for the adsorbent and adsorbate is 60 minutes. This duration achieves an effective degree of purification for ferric ions (82.5 %) and manganese ions (84.65 %). Compared to КАУ coconut coal and other studied adsorbents, the purification rates for iron ions and manganese ions are 7–15 % and 18–20 % higher, respectively. The study determined that the optimal dose of КАУ+АН adsorbent is 5 g/dm3. The advantages of this adsorbent, which is characterized by a high degree of extraction of iron and manganese ions in static conditions and can be recommended for cleaning aqueous solutions and further research in dynamic conditions, are given.
References
Iron and manganese removal from water supplies. Water Treatment Services. https://watertreatmentservices.co.uk/iron-manganese-removal-water-supplies/
Naseem, T., Durrani, T. (2021). The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environmental Chemistry and Ecotoxicology, 3, 59–75. https://doi.org/10.1016/j.enceco.2020.12.001
Dey, S., Tripathy, B., Kumar, M. S., Das, A. P. (2023). Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environmental Chemistry and Ecotoxicology. https://doi.org/10.1016/j.enceco.2023.01.001
Dvorak, B., Schuerman, B. (2021). Drinking water: iron and manganese. Institute of agriculture and natural resources Nebraska extension publications. https://extensionpublications.unl.edu/assets/html/g1714/build/g1714.htm (date of access: 16.05.2023
Voloshyn, M., Vladimirova, V. (2021). [Modern methods of water purification. Modern technologies and achievements of engineering sciences in the field of hydraulic engineering construction and water engineering: a collection of scientific works]. 3rd issue. - Kherson: KhSAEU. (in Ukrainian).
Corbera-Rubio, F., Laureni, M., Koudijs, N., Müller, S., van Alen, T., Schoonenberg, F., Lücker, S., Pabst, M., van Loosdrecht, M. C. M., van Halem, D. (2023). Meta-omics profiling of full-scale groundwater rapid sand filters explains stratification of iron, ammonium and manganese removals. Water Research, 233, 119805. https://doi.org/10.1016/j.watres.2023.119805
Das, S., Mishra, S., Sahu, H. (2023). A review of activated carbon to counteract the effect of iron toxicity on the environment. Environmental Chemistry and Ecotoxicology, 5, 86–97. https://doi.org/10.1016/j.enceco.2023.02.002
Arafat, M., Marzouk, S. Y., El Monayeri, O. D. (2021). Hybrid system for iron and manganese reduction from polluted water using adsorption and filtration. Ain Shams Engineering Journal, 12(3), 2465–2470. https://doi.org/10.1016/j.asej.2021.02.001
Kwadjo Siabi, W., Degraft-Johnson Owusu-Ansah, E., Michelle Korkor Essandoh, H., Yaw Asiedu, N. (2021). Modelling the adsorption of iron and manganese by activated carbon from teak and shea charcoal for continuous low flow. Water-Energy Nexus, 4, 88–94. https://doi.org/10.1016/j.wen.2021.02.001
Fseha, Y. H., Sizirici, B., Yildiz, I. (2022). Manganese and nitrate removal from groundwater using date palm biochar: application for drinking water. Environmental Advances, 100237. https://doi.org/10.1016/j.envadv.2022.100237
Michael, K., Wilson, A. W., Govender, P. P. (2022). Modelling of manganese-contaminated groundwater through batch experiments: Implications for bone char remediation. Environmental Advances, 10, 100323. https://doi.org/10.1016/j.envadv.2022.100323
Gomelya M., Trus I., Tverdokhlib M. (2021). [Study of the efficiency of water purification from iron and manganese ions by magnetite-based sorbents]. Problems of ecology and energy saving: Proceedings of the conference, 11–15 (in Ukrainian)
Rudenko, V., Ivanenko, I. M., Коsogina I., Burmak, A. (2021). [New efficient carbon adsorbent for water deironing]. Bulletin of Cherkasy State Technological University, (1), 144–154. https://doi.org/10.24025/2306-4412.1.2021.225318 (in Ukrainian)/
Pachana, P. K., Rattanasak, U., Nuithitikul, K., Jitsangiam, P., Chindaprasirt, P. (2022). Sustainable utilization of water treatment residue as a porous geopolymer for iron and manganese removals from groundwater. Journal of Environmental Management, 302, 114036. https://doi.org/10.1016/j.jenvman.2021.114036
Solodovnik, T., Yakymenko, I. K. (2021). [Problems and methods of drinking water purification in the systems of decentralized water supply]. Bulletin of Cherkasy State Technological University, (2), 63–81. https://doi.org/10.24025/2306-4412.2.2021.239703 (in Ukrainian).
Solodovnik, T.V., Tolstopalova, N. M., Fomina, N. M., Yakymenko, I. K. (2019). [Study of the processes of colored solutions purification using inorganic coagulants and natural floculant]. Bulletin of Cherkasy State Technological University, (3), 108–117. https://doi.org/10.24025/2306-4412.3.2019.167654 (in Ukrainian).
Solodovnik, T., Yakymenko, I. (2021). [Research and improvement of flocculation-coagulation processes of purification of colored industrial wastes]. Bulletin of Cherkasy State Technological University, (3), 94–102. https://doi.org/10.24025/2306-4412.3.2020.213912 (in Ukrainian)
Mensah-Akutteh, H., Buamah, R., Wiafe, S., Nyarko, K. B. (2022). Optimizing coagulation–flocculation processes with aluminium coagulation using response surface methods. Applied Water Science, 12(8). https://doi.org/10.1007/s13201-022-01708-1
Gao, X., Guo, C., Hao, J., Zhao, Z., Long, H., Li, M. (2020). Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. International Journal of Biological Macromolecules, 164, 4423–4434. https://doi.org/10.1016/j.ijbiomac.2020.09.046
Pontius, W. F. (2016). Chitosan as a drinking water treatment coagulant. American Journal of Civil Engineering, 4(5), 205. https://doi.org/10.11648/j.ajce.20160405.11.
[On the approval of State sanitary norms and rules "Hygienic requirements for drinking water intended for human consumption" (DSanPiN 2.2.4-171-10), Order of the Ministry of Health of Ukraine No. 400 (2022)]. (In Ukrainian). https://zakon.rada.gov.ua/laws/show/z0452-10#Text
Litynska M. (2021). [Removal of arsenic compounds and humates from the water environment]: dis. candidate technical of Science. Kyiv. (in Ukrainian).
Ivanenko I. M., Dontsova T. A., Yu M. Fedenko. (2018) Adsorption, adsorbents and catalysts based on them: textbook for students of specialty 161 "Chemical technologies and engineering" specialization "Chemical technologies of inorganic substances and water treatment". Kyiv : KPI named after Igor Sikorsky, 233 p. (in Ukrainian)
Astrelin, I., Ratnavira, H. (Eds.). (2015). Physical and chemical methods of water purification. Management of water resources. "Drukarnia Wolf" LLC. (in Ukrainian).
Andrusyshina I.M., Golub I.O., Lampeka O.G. (2018) Manganese in water is a dangerous pollutant All-Ukrainian "WaterNet" water company. Kyiv: UVO "WaterNet" (in Ukrainian)
Yakymenko, I. K., Solodovnik, T. V. (2023). [Adsorption materials for additional cleaning of drinking water from iron and manganese compounds in decentralized water supply systems]. Scientific Notes of Taurida National V.I. Vernadsky University. Series: Technical Sciences, 2(2), 72–77. https://doi.org/10.32782/2663-5941/2023.2.2/13 (in Ukrainian).
Downloads
Published
Issue
Section
License
Copyright (c) 2024 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

